8-EpixanthatinCAS# 30890-35-8 |
- Xanthatin
Catalog No.:BCN5150
CAS No.:26791-73-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 30890-35-8 | SDF | Download SDF |
| PubChem ID | 11694445 | Appearance | Powder |
| Formula | C15H18O3 | M.Wt | 246.30 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
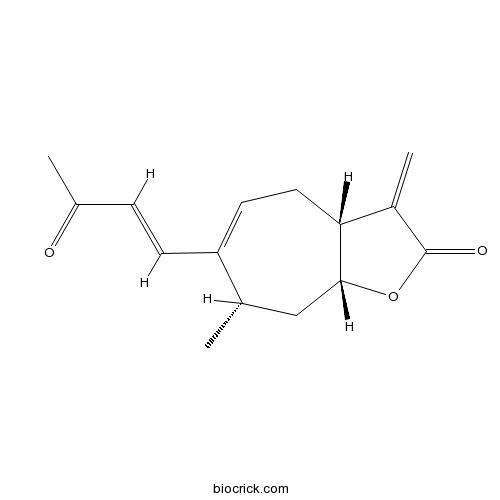
| Chemical Name | (3aR,7S,8aR)-7-methyl-3-methylidene-6-[(E)-3-oxobut-1-enyl]-4,7,8,8a-tetrahydro-3aH-cyclohepta[b]furan-2-one | ||
| SMILES | CC1CC2C(CC=C1C=CC(=O)C)C(=C)C(=O)O2 | ||
| Standard InChIKey | RBRPTFMVULVGIC-MDKNCZOUSA-N | ||
| Standard InChI | InChI=1S/C15H18O3/c1-9-8-14-13(11(3)15(17)18-14)7-6-12(9)5-4-10(2)16/h4-6,9,13-14H,3,7-8H2,1-2H3/b5-4+/t9-,13+,14+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 8-Epixanthatin can induce germination of O. cumana at nano- to micromolar concentrations. 2. 8-Epixanthatin, a light-induced growth inhibitor, mediates the phototropic curvature in sunflower (Helianthus annuus) hypocotyls. 3. 8-Epixanthatin has antiprotozoal activity. |
| Targets | Antifection |

8-Epixanthatin Dilution Calculator

8-Epixanthatin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0601 mL | 20.3004 mL | 40.6009 mL | 81.2018 mL | 101.5022 mL |
| 5 mM | 0.812 mL | 4.0601 mL | 8.1202 mL | 16.2404 mL | 20.3004 mL |
| 10 mM | 0.406 mL | 2.03 mL | 4.0601 mL | 8.1202 mL | 10.1502 mL |
| 50 mM | 0.0812 mL | 0.406 mL | 0.812 mL | 1.624 mL | 2.03 mL |
| 100 mM | 0.0406 mL | 0.203 mL | 0.406 mL | 0.812 mL | 1.015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Kauran-18-Olc Acid,16,1719-Tnhydroxy-,(4A)
Catalog No.:BCC9235
CAS No.:308821-59-2
- Aloesin
Catalog No.:BCN8437
CAS No.:30861-27-9
- PM00104
Catalog No.:BCC4237
CAS No.:308359-57-1
- Spongouridine
Catalog No.:BCC9152
CAS No.:3083-77-0
- 4-Oxododecanedioic acid
Catalog No.:BCN5223
CAS No.:30828-09-2
- AEBSF.HCl
Catalog No.:BCC1219
CAS No.:30827-99-7
- L-Theanine
Catalog No.:BCN2571
CAS No.:3081-61-6
- beta-Anhydrouzarigenin
Catalog No.:BCN5222
CAS No.:3080-20-4
- [Des-octanoyl]-Ghrelin (rat)
Catalog No.:BCC5953
CAS No.:307950-60-3
- Crosemperine
Catalog No.:BCN2074
CAS No.:30785-56-9
- STF 083010
Catalog No.:BCC6209
CAS No.:307543-71-1
- SMER 28
Catalog No.:BCC7908
CAS No.:307538-42-7
- 3β-Acetoxy-5α-androstan-17β-ol
Catalog No.:BCC8644
CAS No.:3090-70-8
- Boc-N-Me-Phg-OH
Catalog No.:BCC3350
CAS No.:30925-11-2
- Boc-Asp-OBzl
Catalog No.:BCC3363
CAS No.:30925-18-9
- Inauhzin
Catalog No.:BCC5146
CAS No.:309271-94-1
- Doxifluridine
Catalog No.:BCC4903
CAS No.:3094-09-5
- Perillartine
Catalog No.:BCN8305
CAS No.:30950-27-7
- 2-Amino-9H-fluoren-9-one
Catalog No.:BCC8545
CAS No.:3096-57-9
- 1,5-Dicaffeoylquinic acid
Catalog No.:BCN5913
CAS No.:30964-13-7
- GW791343 HCl
Catalog No.:BCC4974
CAS No.:309712-55-8
- Vinyl Cinnamate
Catalog No.:BCN5041
CAS No.:3098-92-8
- Dauricinoline
Catalog No.:BCC8162
CAS No.:30984-80-6
- SX 011
Catalog No.:BCC7731
CAS No.:309913-42-6
The antiprotozoal activity of sixteen asteraceae species native to Sudan and bioactivity-guided isolation of xanthanolides from Xanthium brasilicum.[Pubmed:19431098]
Planta Med. 2009 Oct;75(12):1363-8.
In vitro screening of the dichloromethane extracts of 16 Asteraceae species native to Sudan for activity against major protozoan pathogens revealed that a Xanthium brasilicum Vell. [syn. X. strumarium var. brasilicum (Vell.) Baker in Mart.] extract was the most active against Trypanosoma brucei rhodesiense, the etiological agent of East African human trypanosomiasis (IC(50) = 0.1 microg/mL). This plant extract also exhibited noticeable activities against T. cruzi (Chagas disease), Leishmania donovani (Kala-Azar) as well as Plasmodium falciparum (Malaria tropica). Bioactivity-guided fractionation resulted in the isolation of four bioactive sesquiterpene lactones (STL) of the xanthanolide series (4,5-seco-guaianolide-type). They were identified by spectroscopic means as 8-Epixanthatin (1), 8-Epixanthatin 1beta,5beta-epoxide (2), and as the dimers pungiolide A (4) as well as pungiolide B (5). Two further modified xanthanolide sesquiterpene lactones, xanthipungolide (3) and 4,15-dinor-1,11(13)-xanthadiene-3,5beta:12,8beta-diolide (6) were isolated. While xanthipungolide turned out to be inactive against the tested parasites, the dinor-xanthanlide showed significant activity against T. brucei rhodesiense and L. donovani. All isolated compounds were previously known from other Xanthium species but this is the first report on their occurrence in X. brasilicum, and, most notably, on their antiprotozoal activity. As the most active single compound from this extract, 8-Epixanthatin 1beta,5beta-epoxide showed IC(50) values of 0.09, 2.95, 0.16 and 1.71 microg/mL (0.33, 11.3, 0.6 and 6.5 microM) against T. brucei rhodesiense, T. cruzi, L. donovani and P. falciparum, respectively, while its cytotoxicity against rat myoblast cells used as control was determined at 5.8 microg/mL (22.1 microM). Besides assessment of their antiprotozoal activity, the structural assignments for the dimeric xanthanolides pungiolide A and B were reinvestigated and fully established.
New sesquiterpene lactones from sunflower root exudate as germination stimulants for Orobanche cumana.[Pubmed:24117219]
J Agric Food Chem. 2013 Nov 6;61(44):10481-7.
Orobanche cumana is a serious threat for cultivation of sunflower in Europe and Asia. Germination of the parasite is induced by metabolites released from the host root system. The first germination stimulant from sunflower root exudate was recently identified as dehydrocostus lactone, a sesquiterpene lactone. Bioassay-guided fractionation of root exudates now showed the release of additional sesquiterpene lactones. Besides dehydrocostus lactone, costunolide, tomentosin, and 8-Epixanthatin were purified and identified spectroscopically. All four compounds induced germination of O. cumana at nano- to micromolar concentrations. Costunolide and dehydrocostus lactone concentrations above 1 muM reduced the activity, and application of 100 muM inhibited germination irreversibly. Seeds of Phelipanche ramosa could not be induced with costunolide. O. cumana seeds also germinated with GR24, a synthetic strigolactone. No bioactive fraction of sunflower contained compounds of this type. This supports previous findings that sesquiterpene lactones instead of strigolactones trigger the sunflower/O. cumana interaction.


