PerillartineCAS# 30950-27-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 30950-27-7 | SDF | Download SDF |
| PubChem ID | 5365782 | Appearance | Colorless columnar crystal |
| Formula | C10H15NO | M.Wt | 165.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | DL-Perillartine | ||
| Solubility | DMSO : ≥ 100 mg/mL (605.22 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
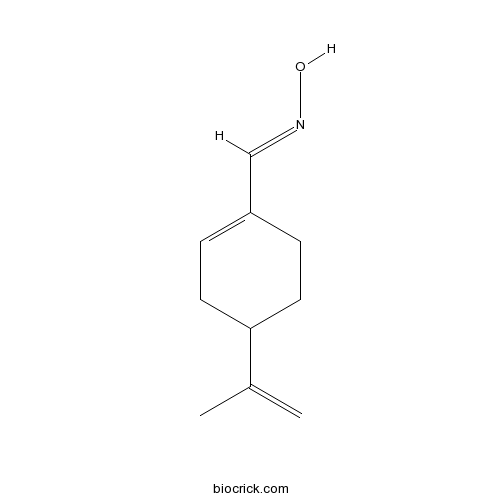
| Chemical Name | (NE)-N-[(4-prop-1-en-2-ylcyclohexen-1-yl)methylidene]hydroxylamine | ||
| SMILES | CC(=C)C1CCC(=CC1)C=NO | ||
| Standard InChIKey | XCOJIVIDDFTHGB-YRNVUSSQSA-N | ||
| Standard InChI | InChI=1S/C10H15NO/c1-8(2)10-5-3-9(4-6-10)7-11-12/h3,7,10,12H,1,4-6H2,2H3/b11-7+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Perillartine Dilution Calculator

Perillartine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.0522 mL | 30.2608 mL | 60.5217 mL | 121.0434 mL | 151.3042 mL |
| 5 mM | 1.2104 mL | 6.0522 mL | 12.1043 mL | 24.2087 mL | 30.2608 mL |
| 10 mM | 0.6052 mL | 3.0261 mL | 6.0522 mL | 12.1043 mL | 15.1304 mL |
| 50 mM | 0.121 mL | 0.6052 mL | 1.2104 mL | 2.4209 mL | 3.0261 mL |
| 100 mM | 0.0605 mL | 0.3026 mL | 0.6052 mL | 1.2104 mL | 1.513 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Perillartine is a sweetener, which activates the taste receptor type 1 member 2 (Tas1r2) subunit in a species-dependent manner.
In Vitro:The responses of the monomeric Tas1r2 subunits of human, rhesus monkey, squirrel monkey and mouse to Perillartine are examined, respectively. The human, rhesus monkey and squirrel monkey Tas1r2 subunits can be activated by Perillartine, while mouse Tas1r2 can not. The insensitivity of human, rhesus monkey, squirrel monkey and mouse Tas1r2 subunits to cyclamate precludes the probable involvement of Tas1r3 subunit in the assay. Replacement of the mouse Tas1r2 with rhesus monkey Tas1r2 (rhTas1r2/mTas1r3) leads to a gain of response to Perillartine. The dose-response curve show the efficacy of responses of the Tas1r2 subunits among species: hTAS1R2>rhTas1r2>smTas1r2>mTas1r2. These results demonstrate that the monomeric Tas1r2 subunit can be activated by Perillartine in a species-dependent manner[1].
References:
[1]. Cai C, et al. Characterization of the Sweet Taste Receptor Tas1r2 from an Old World Monkey Species Rhesus Monkey and Species-Dependent Activation of the Monomeric Receptor by an Intense SweetenerPerillartine. PLoS One. 2016 Aug 1;11(8):e0160079.
- Doxifluridine
Catalog No.:BCC4903
CAS No.:3094-09-5
- Inauhzin
Catalog No.:BCC5146
CAS No.:309271-94-1
- Boc-Asp-OBzl
Catalog No.:BCC3363
CAS No.:30925-18-9
- Boc-N-Me-Phg-OH
Catalog No.:BCC3350
CAS No.:30925-11-2
- 3β-Acetoxy-5α-androstan-17β-ol
Catalog No.:BCC8644
CAS No.:3090-70-8
- 8-Epixanthatin
Catalog No.:BCN7782
CAS No.:30890-35-8
- Kauran-18-Olc Acid,16,1719-Tnhydroxy-,(4A)
Catalog No.:BCC9235
CAS No.:308821-59-2
- Aloesin
Catalog No.:BCN8437
CAS No.:30861-27-9
- PM00104
Catalog No.:BCC4237
CAS No.:308359-57-1
- Spongouridine
Catalog No.:BCC9152
CAS No.:3083-77-0
- 4-Oxododecanedioic acid
Catalog No.:BCN5223
CAS No.:30828-09-2
- AEBSF.HCl
Catalog No.:BCC1219
CAS No.:30827-99-7
- 2-Amino-9H-fluoren-9-one
Catalog No.:BCC8545
CAS No.:3096-57-9
- 1,5-Dicaffeoylquinic acid
Catalog No.:BCN5913
CAS No.:30964-13-7
- GW791343 HCl
Catalog No.:BCC4974
CAS No.:309712-55-8
- Vinyl Cinnamate
Catalog No.:BCN5041
CAS No.:3098-92-8
- Dauricinoline
Catalog No.:BCC8162
CAS No.:30984-80-6
- SX 011
Catalog No.:BCC7731
CAS No.:309913-42-6
- Boc-Aib-OH
Catalog No.:BCC3148
CAS No.:30992-29-1
- KL 001
Catalog No.:BCC6262
CAS No.:309928-48-1
- 3-(Boc-Amino)piperidine
Catalog No.:BCC8590
CAS No.:309956-78-3
- 7-O-Methylmangiferin
Catalog No.:BCN2804
CAS No.:31002-12-7
- 1-O-(3,4-Dimethoxybenzoyl)-beta-D-glucopyranose
Catalog No.:BCN3759
CAS No.:31002-27-4
- 7-Ethoxycoumarin
Catalog No.:BCN2708
CAS No.:31005-02-4
A consideration for structure-taste correlations of perillartines using pattern-recognition techniques.[Pubmed:7143365]
J Med Chem. 1982 Oct;25(10):1245-8.
The relationships between molecular structure and taste quality (sweet or bitter) or several Perillartine derivatives were investigated using pattern-recognition techniques. For the classification of these compounds into two classes (sweet or bitter), a significant discriminant function was developed by the use of linear learning machine. All the compounds were assigned correctly to their observed taste classes by the function involving three parameters (one hydrophobic and two steric). In addition, the K-L transformation technique was used for examination of classification results.
Structure-activity maps for visualizing the graph variables arising in drug design.[Pubmed:8220404]
J Biopharm Stat. 1993 Sep;3(2):203-36.
Structure-activity problems are characterized by the topological and topographical character of the structural information determining the activity. Traditional statistical methodology requires that this predictive information be mapped to a vector space. To circumvent this vexing conversion of structural information to vector form, the edge-deletion metric is defined on the space of chemical graphs that defines the topology of the molecules. This paper proposes structure-activity maps and transformation-effect maps for directly visualizing the structure-activity relationships. The maps are illustrated using the hypotensive activities of clonidine analogs and the sweet taste of Perillartine analogs.
Structure--taste relationship of perillartine and nitro- and cyanoaniline derivatives.[Pubmed:7365747]
J Med Chem. 1980 Mar;23(3):308-12.
The relationship between structure and taste potency of Perillartine and its analogues was investigated quantitatively by physicochemical parameters and regression analysis. The results indicated that the hydrophobicity estimated from the 1-octanol/water partition coefficient and the molecular widths from the bond axis connecting the oxime carbon and alicyclic ring are important, regardless of whether the taste is sweet or bitter, so far as the taste potency is concerned. The SAR for the sweet/bitter ratio was not established quantitatively, but the molecular width and thickness and the position-specific electronic effect seem to delineate the ratio qualitatively; i.e., in principle, the wider and/or the thicker the molecule, the more bitter the taste. Comparatively, the QSAR of 5-nitro- and 5-cyanoaniline sweetners was formulated to show the insignificance of the hydrophobicity within the compounds investigated but the importance of the steric dimensions in determining the activity.
An Overview of Chemical Profiles, Antioxidant and Antimicrobial Activities of Commercial Vegetable Edible Oils Marketed in Japan.[Pubmed:29439420]
Foods. 2018 Feb 10;7(2). pii: foods7020021.
This study analyzed chemical components and investigated the antioxidant and antimicrobial activities of fourteen vegetable edible oils marketed in Japan. High-performance liquid chromatography (HPLC) was used to identify and quantify principal phenolic acids and flavonoids. In the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay, sunflower, safflower, canola, soybean, Inca inchi, sesame, and rice bran showed markedly greater activity, whilst the percentage of lipid peroxidation inhibition (LPI%) in sunflower, canola, cotton, grape, flax, perilla, Inca inchi, Perillartine, and rice bran were significantly higher than other oils. Maximum total phenol content (TPC) was recorded in flax, followed by Perillartine, rice bran, and perilla, whereas total flavonoid content (TFC) was the greatest in Inca inchi and sesame. Benzoic acid was the most common constituent, followed by vanillic acid, p-hydroxybenzoic acid, ferulic acid, and p-coumaric acid. On the other hand, luteolin was the most abundant flavonoid, followed by esculetin, myricetin, isoquercetin, and kaempferol, while fisetin was detected only in sunflower. In general, all of the edible oils showed antimicrobial activity, but the growth inhibition of Staphylococcus aureus and Escherichia coli of cotton, grape, chia, sesame, and rice bran were greater than other oils.
Discriminative structural analysis using pattern recognition techniques in the structure-taste problem of perillartines.[Pubmed:6737255]
J Pharm Sci. 1984 Jun;73(6):737-41.
Pattern recognition techniques have been applied to the study of structure-taste correlations for Perillartine derivatives. The structure of each compound was described by hydrophobicity (log P), logarithm of water solubility (log S), and topological descriptors relating to some positions which were assigned by superposing each compound on a "template" structure. The fragment molecular connectivities were calculated as the topological descriptors. The discriminant functions between the sweet and bitter taste classes were computed by the use of the simplex optimization technique, which correctly recognized most of the compounds under investigation. It was found that the hydrophobicity and one or two topological descriptors concerned with a specific part of the molecules contributed significantly to the discrimination. The discriminant function obtained correctly classified seven of nine compounds (which were not involved in the data set for developing the function) into the taste class to which they belonged.
Ab initio molecular electrostatic potentials of perillartine analogues: implications for sweet-taste receptor recognition.[Pubmed:3172123]
J Med Chem. 1988 Oct;31(10):1879-85.
A model for the recognition of the Perillartine analogues has been determined from a consideration of the molecular electrostatic potentials calculated at the ab initio 3-21G level for a select set of biologically active analogues. The model stresses the importance of two regions of negative electrostatic potential. One region, near the oxime moiety, does not vary in shape or value with substitution in the hydrocarbon domain. A second region in the hydrocarbon domain varies in depth, extension, orientation, and shape, depending on the nature of the substituent. The depth, relative position, and orientation of this latter region in the most potent systems (the 1,4-cyclohexadiene analogue and its p-methyl derivative) serve as the basis for the optimum recognition pattern of these analogues. The rank order of taste potencies is in general agreement with predictions based on this model. In addition, some conclusions are drawn concerning the receptor-analogue interaction as well as the electrostatic features of the receptor.
Characterization of the Sweet Taste Receptor Tas1r2 from an Old World Monkey Species Rhesus Monkey and Species-Dependent Activation of the Monomeric Receptor by an Intense Sweetener Perillartine.[Pubmed:27479072]
PLoS One. 2016 Aug 1;11(8):e0160079.
Sweet state is a basic physiological sensation of humans and other mammals which is mediated by the broadly acting sweet taste receptor-the heterodimer of Tas1r2 (taste receptor type 1 member 2) and Tas1r3 (taste receptor type 1 member 3). Various sweeteners interact with either Tas1r2 or Tas1r3 and then activate the receptor. In this study, we cloned, expressed and functionally characterized the taste receptor Tas1r2 from a species of Old World monkeys, the rhesus monkey. Paired with the human TAS1R3, it was shown that the rhesus monkey Tas1r2 could respond to natural sugars, amino acids and their derivates. Furthermore, similar to human TAS1R2, rhesus monkey Tas1r2 could respond to artificial sweeteners and sweet-tasting proteins. However, the responses induced by rhesus monkey Tas1r2 could not be inhibited by the sweet inhibitor amiloride. Moreover, we found a species-dependent activation of the Tas1r2 monomeric receptors of human, rhesus monkey and squirrel monkey but not mouse by an intense sweetener Perillartine. Molecular modeling and sequence analysis indicate that the receptor has the conserved domains and ligand-specific interactive residues, which have been identified in the characterized sweet taste receptors up to now. This is the first report of the functional characterization of sweet taste receptors from an Old World monkey species.


