DauricinolineCAS# 30984-80-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 30984-80-6 | SDF | Download SDF |
| PubChem ID | 71463990 | Appearance | Powder |
| Formula | C37H42N2O6 | M.Wt | 610.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
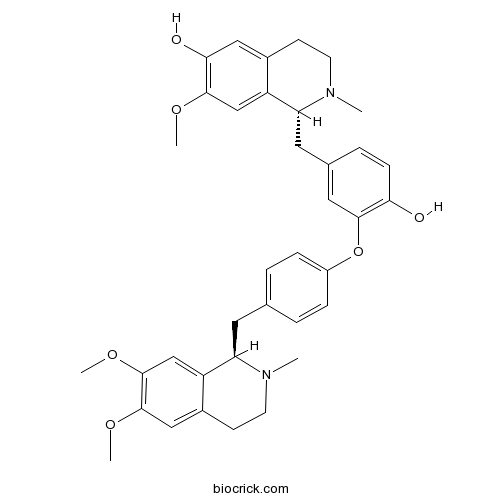
| Chemical Name | (1R)-1-[[3-[4-[[(1R)-6,7-dimethoxy-2-methyl-3,4-dihydro-1H-isoquinolin-1-yl]methyl]phenoxy]-4-hydroxyphenyl]methyl]-7-methoxy-2-methyl-3,4-dihydro-1H-isoquinolin-6-ol | ||
| SMILES | CN1CCC2=CC(=C(C=C2C1CC3=CC=C(C=C3)OC4=C(C=CC(=C4)CC5C6=CC(=C(C=C6CCN5C)O)OC)O)OC)OC | ||
| Standard InChIKey | OMFKIOLXDGQKCF-FIRIVFDPSA-N | ||
| Standard InChI | InChI=1S/C37H42N2O6/c1-38-15-13-26-20-36(43-4)37(44-5)22-29(26)30(38)16-23-6-9-27(10-7-23)45-35-18-24(8-11-32(35)40)17-31-28-21-34(42-3)33(41)19-25(28)12-14-39(31)2/h6-11,18-22,30-31,40-41H,12-17H2,1-5H3/t30-,31-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Dauricinoline Dilution Calculator

Dauricinoline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6375 mL | 8.1873 mL | 16.3747 mL | 32.7493 mL | 40.9366 mL |
| 5 mM | 0.3275 mL | 1.6375 mL | 3.2749 mL | 6.5499 mL | 8.1873 mL |
| 10 mM | 0.1637 mL | 0.8187 mL | 1.6375 mL | 3.2749 mL | 4.0937 mL |
| 50 mM | 0.0327 mL | 0.1637 mL | 0.3275 mL | 0.655 mL | 0.8187 mL |
| 100 mM | 0.0164 mL | 0.0819 mL | 0.1637 mL | 0.3275 mL | 0.4094 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Vinyl Cinnamate
Catalog No.:BCN5041
CAS No.:3098-92-8
- GW791343 HCl
Catalog No.:BCC4974
CAS No.:309712-55-8
- 1,5-Dicaffeoylquinic acid
Catalog No.:BCN5913
CAS No.:30964-13-7
- 2-Amino-9H-fluoren-9-one
Catalog No.:BCC8545
CAS No.:3096-57-9
- Perillartine
Catalog No.:BCN8305
CAS No.:30950-27-7
- Doxifluridine
Catalog No.:BCC4903
CAS No.:3094-09-5
- Inauhzin
Catalog No.:BCC5146
CAS No.:309271-94-1
- Boc-Asp-OBzl
Catalog No.:BCC3363
CAS No.:30925-18-9
- Boc-N-Me-Phg-OH
Catalog No.:BCC3350
CAS No.:30925-11-2
- 3β-Acetoxy-5α-androstan-17β-ol
Catalog No.:BCC8644
CAS No.:3090-70-8
- 8-Epixanthatin
Catalog No.:BCN7782
CAS No.:30890-35-8
- Kauran-18-Olc Acid,16,1719-Tnhydroxy-,(4A)
Catalog No.:BCC9235
CAS No.:308821-59-2
- SX 011
Catalog No.:BCC7731
CAS No.:309913-42-6
- Boc-Aib-OH
Catalog No.:BCC3148
CAS No.:30992-29-1
- KL 001
Catalog No.:BCC6262
CAS No.:309928-48-1
- 3-(Boc-Amino)piperidine
Catalog No.:BCC8590
CAS No.:309956-78-3
- 7-O-Methylmangiferin
Catalog No.:BCN2804
CAS No.:31002-12-7
- 1-O-(3,4-Dimethoxybenzoyl)-beta-D-glucopyranose
Catalog No.:BCN3759
CAS No.:31002-27-4
- 7-Ethoxycoumarin
Catalog No.:BCN2708
CAS No.:31005-02-4
- Magnolin
Catalog No.:BCN5224
CAS No.:31008-18-1
- Fargesin
Catalog No.:BCN5022
CAS No.:31008-19-2
- Dihydrosphingosine
Catalog No.:BCC6778
CAS No.:3102-56-5
- Ceramide
Catalog No.:BCC2458
CAS No.:3102-57-6
- Echinophyllin C
Catalog No.:BCN5225
CAS No.:310433-44-4
Identification of nuclear factor-kappaB inhibitors in the folk herb Rhizoma Menispermi via bioactivity-based ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry analysis.[Pubmed:25252930]
BMC Complement Altern Med. 2014 Sep 25;14:356.
BACKGROUND: Rhizoma Menispermi (RM) is the dried root of Menispermum dauricum DC, which is traditionally used to treat swelling and pain for sore throat, enteritis and rheumatic arthralgia in the clinic, but its bioactive compounds remain unclear. METHODS: In this study, RM extract was administered orally to ICR mice followed by challenging with an intratracheal Pseudomonas aeruginosa suspension. Then mortality, histological features of lung, and inflammatory cytokines were evaluated. RM treatment significantly ameliorated Pseudomonas aeruginosa-induced acute lung inflammation and reduced levels of inflammatory mediators. To screen for potential anti-inflammatory constituents of the RM extract, a simple and rapid method based on ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry (UPLC-Q/TOF MS) coupled with a luciferase reporter assay system to detect nuclear factor-kappaB (NF-kappaB) activity was established. RESULTS: Using this system, seven potential NF-kappaB inhibitors were detected, including sinomenine, norsinoacutin, N-norsinoacutin-beta-D-glucopyranoside, 6-O-methyl-laudanosoline-13-O-glucopyranoside, magnoflorine, laurifloline and Dauricinoline. Furthermore, IL-6 and IL-8 assays confirmed the anti-inflammatory effects of these potential NF-kappaB inhibitors, in which norsinoacutin, 6-O-methyl-laudanosoline-13-O-glucopyranoside laurifloline, Dauricinoline and N-norsinoacutin-beta-D-glucopyranoside were revealed as new NF-kappaB inhibitors. CONCLUSION: This method of UPLC-Q/TOF coupled with the luciferase reporter assay system was initially applied to the study of RM and was demonstrated to represent a simple, rapid and practical approach to screen for anti-inflammatory compounds. This study provided useful results for further investigation on the anti-inflammatory mechanism of RM.
The interaction of telomeric DNA and C-myc22 G-quadruplex with 11 natural alkaloids.[Pubmed:22480315]
Nucleic Acid Ther. 2012 Apr;22(2):127-36.
Telomeric DNA and C-myc22 are DNA G-quadruplex (G4)-forming sequences associated with tumorigenesis. Ligands that can facilitate the formation and increase the stabilization of G4 can halt tumor cell proliferation and have been regarded as potential anti-cancer drugs. In the present study, we have investigated the interaction of 11 natural alkaloids with G4 formed by telomeric DNA and C-myc22 sequences. Our results indicated that sanguinarine (San), palmatine (Pal), and berberine (Beb) of the first series (S1) can induce the formation of G4 as well as increase the stabilization ability. Daurisoline (S2-1), O-methyldauricine (S2-2), O-diacetyldaurisoline (S2-3), daurinoline (S2-4), Dauricinoline (S2-5), N,N'-dimethyldauricine iodide (S2-6), and N,N'-dimethyldaurisoline iodide (S2-7) of the second series (S2) showed similar stabilization ability. We found that unsaturated ring C, N(+) positively charged centers, and conjugated aromatic rings are key factors to increase the stabilization ability of S1, and we gave some advice on structure modification to S2 through structure-activity study. Besides, we found San and Pal to be cell cycle blocker in G(1). San was speculated to bind to G4 through intercalation or end stacking.
[Isolation and identification of alkaloids form Menispermum dauricum growing in Xianning].[Pubmed:12569837]
Zhong Yao Cai. 1998 Sep;21(9):456-8.
The alkaloids of rhizoma of Menispermum dauricum DC growing in Xianning have been subjected to isolation and identification. The results showed that its two major constituents, which are only next of dauricine in content, are Dauricinoline and daurinoline, instead of the commonly found daurisoline in the same plant materials from North China.


