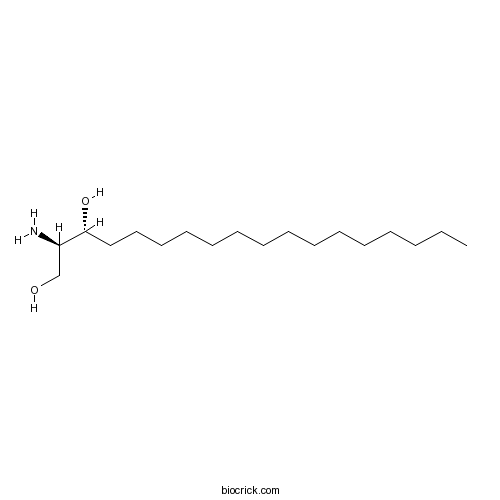
DihydrosphingosineProtein kinase C inhibitor CAS# 3102-56-5 |
- MLN9708
Catalog No.:BCC2091
CAS No.:1201902-80-8
- MG-115
Catalog No.:BCC1237
CAS No.:133407-86-0
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- CEP-18770
Catalog No.:BCC2093
CAS No.:847499-27-8
- Carfilzomib (PR-171)
Catalog No.:BCC1145
CAS No.:868540-17-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3102-56-5 | SDF | Download SDF |
| PubChem ID | 5746414 | Appearance | Powder |
| Formula | C18H39NO2 | M.Wt | 301.51 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Sphinganine | ||
| Solubility | Soluble to 100 mM in DMSO | ||
| Chemical Name | (2R,3R)-2-aminooctadecane-1,3-diol | ||
| SMILES | CCCCCCCCCCCCCCCC(C(CO)N)O | ||
| Standard InChIKey | OTKJDMGTUTTYMP-QZTJIDSGSA-N | ||
| Standard InChI | InChI=1S/C18H39NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(21)17(19)16-20/h17-18,20-21H,2-16,19H2,1H3/t17-,18-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Dihydrosphingosine Dilution Calculator

Dihydrosphingosine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3166 mL | 16.5832 mL | 33.1664 mL | 66.3328 mL | 82.916 mL |
| 5 mM | 0.6633 mL | 3.3166 mL | 6.6333 mL | 13.2666 mL | 16.5832 mL |
| 10 mM | 0.3317 mL | 1.6583 mL | 3.3166 mL | 6.6333 mL | 8.2916 mL |
| 50 mM | 0.0663 mL | 0.3317 mL | 0.6633 mL | 1.3267 mL | 1.6583 mL |
| 100 mM | 0.0332 mL | 0.1658 mL | 0.3317 mL | 0.6633 mL | 0.8292 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fargesin
Catalog No.:BCN5022
CAS No.:31008-19-2
- Magnolin
Catalog No.:BCN5224
CAS No.:31008-18-1
- 7-Ethoxycoumarin
Catalog No.:BCN2708
CAS No.:31005-02-4
- 1-O-(3,4-Dimethoxybenzoyl)-beta-D-glucopyranose
Catalog No.:BCN3759
CAS No.:31002-27-4
- 7-O-Methylmangiferin
Catalog No.:BCN2804
CAS No.:31002-12-7
- 3-(Boc-Amino)piperidine
Catalog No.:BCC8590
CAS No.:309956-78-3
- KL 001
Catalog No.:BCC6262
CAS No.:309928-48-1
- Boc-Aib-OH
Catalog No.:BCC3148
CAS No.:30992-29-1
- SX 011
Catalog No.:BCC7731
CAS No.:309913-42-6
- Dauricinoline
Catalog No.:BCC8162
CAS No.:30984-80-6
- Vinyl Cinnamate
Catalog No.:BCN5041
CAS No.:3098-92-8
- GW791343 HCl
Catalog No.:BCC4974
CAS No.:309712-55-8
- Ceramide
Catalog No.:BCC2458
CAS No.:3102-57-6
- Echinophyllin C
Catalog No.:BCN5225
CAS No.:310433-44-4
- Spaglumic acid
Catalog No.:BCC6632
CAS No.:3106-85-2
- Cedeodarin
Catalog No.:BCN4784
CAS No.:31076-39-8
- Alytesin
Catalog No.:BCC7203
CAS No.:31078-12-3
- Decylic acid vanillylamide
Catalog No.:BCN7836
CAS No.:31078-36-1
- PRT 4165
Catalog No.:BCC6354
CAS No.:31083-55-3
- 5,7,3'-Trihydroxy-6,4',5'-trimethoxyflavanone
Catalog No.:BCN1461
CAS No.:310888-07-4
- Benzoquinonium dibromide
Catalog No.:BCC6641
CAS No.:311-09-1
- Taxifolin 3'-O-glucoside
Catalog No.:BCN6808
CAS No.:31106-05-5
- 3-Methyl-4-nitrobenzoic acid
Catalog No.:BCN2261
CAS No.:3113-71-1
- RFRP 3 (human)
Catalog No.:BCC6261
CAS No.:311309-27-0
Scintillation Proximity Assay to Detect the Changes in Cellular Dihydrosphingosine 1-Phosphate Levels.[Pubmed:27585475]
Lipids. 2016 Oct;51(10):1207-1216.
Compounds that modulate the activity of sphingosine 1-phosphate (S1P)-metabolizing enzymes are expected to be potential therapeutic agents for various diseases. Investigation of their potencies requires not only cell-free but also cell-based assays in which intracellular accumulation/depletion of S1P could be monitored. However, conventional methods have limitations to their simplicity, mainly due to the necessity of a separation process that separates S1P from its related substances. Here, we describe a method utilizing a scintillation proximity assay (SPA) for semi-quantifying intracellular [(3)H]-labeled dihydroS1P ([(3)H]dhS1P), which is also a substrate for S1P-metabolizing enzymes. We found that uncoated yttrium silicate SPA beads could selectively bind to and detect [(3)H]dhS1P rather than [(3)H]Dihydrosphingosine (the non-phosphorylated form of [(3)H]dhS1P). Based on this, we developed a novel cell-based assay system which does not require any organic solvent extraction or chromatographic separation, and confirmed its practicality by using siRNA targeting S1P lyase (S1PL) and known S1PL inhibitors as models. Our results demonstrated that this assay is useful for rapid and easy evaluation of S1PL inhibitors, and could be potentially applicable for all compounds that modulate the activity of S1P-metabolizing enzymes.
Efficacious In Vitro and In Vivo Effects of Dihydrosphingosine-Ethambutol Analogues Against Susceptible and Multi-drug-resistant Mycobacterium tuberculosis.[Pubmed:27664485]
Arch Med Res. 2016 May;47(4):262-70.
BACKGROUND AND AIMS: Tuberculosis (TB) is a major worldwide health problem in part due to the lack of new drugs and the emergence of multidrug-resistant strains (MDR). The aim of this study was to select anti-tuberculosis drug candidates from a collection of 69 synthetic sphingosine-ethambutol analogues through in vitro and in vivo evaluations. METHODS: The 69 compounds were evaluated in vitro against two Mycobacterium tuberculosis strains, a drug susceptible (H37Rv) and a MDR clinical isolate (CIBIN-99). Four selected compounds, those that exhibited the highest potency in vitro, were tested in vivo using a model of progressive TB in BALB/c mice infected with the drug susceptible strain, either alone or combined with conventional chemotherapy, as well as in mice infected with the MDR strain. The acute toxicity was evaluated on male and female adult BALB/c mice. RESULTS: Ten of the evaluated compounds resulted more potent in vitro than ethambutol. The experimental compound 2b (2-aminopalmitol benzyl ether) was the most efficacious and also showed additive effects in combination with conventional chemotherapy. It did not exhibit toxicity (LD50 >2000 mg/kg). CONCLUSIONS: Compound 2b can be considered as a new drug candidate to continue its development against M. tuberculosis MDR strains.
Phytosphingosine, sphingosine and dihydrosphingosine ceramides in model skin lipid membranes: permeability and biophysics.[Pubmed:28109750]
Biochim Biophys Acta Biomembr. 2017 May;1859(5):824-834.
Ceramides based on phytosphingosine, sphingosine and Dihydrosphingosine are essential constituents of the skin lipid barrier that protects the body from excessive water loss. The roles of the individual ceramide subclasses in regulating skin permeability and the reasons for C4-hydroxylation of these sphingolipids are not completely understood. We investigated the chain length-dependent effects of dihydroceramides, sphingosine ceramides (with C4-unsaturation) and phytoceramides (with C4-hydroxyl) on the permeability, lipid organization and thermotropic behavior of model stratum corneum lipid membranes composed of ceramide/lignoceric acid/cholesterol/cholesteryl sulfate. Phytoceramides with very long C24 acyl chains increased the permeability of the model lipid membranes compared to dihydroceramides or sphingosine ceramides with the same chain lengths. Either unsaturation or C4-hydroxylation of dihydroceramides induced chain length-dependent increases in membrane permeability. Infrared spectroscopy showed that C4-hydroxylation of the sphingoid base decreased the relative ratio of orthorhombic chain packing in the membrane and lowered the miscibility of C24 phytoceramide with lignoceric acid. The phase separation in phytoceramide membranes was confirmed by X-ray diffraction. In contrast, phytoceramides formed strong hydrogen bonds and highly thermostable domains. Thus, the large heterogeneity in ceramide structures and in their aggregation mechanisms may confer resistance towards the heterogeneous external stressors that are constantly faced by the skin barrier.
Inhibitory Effect of Dihydrosphingosine with alpha-Tocopherol on Volatile Formation during the Autoxidation of Polyunsaturated Triacylglycerols.[Pubmed:27477074]
J Oleo Sci. 2016 Sep 1;65(9):713-22.
The effect of Dihydrosphingosine (d18:0) on triacylglycerol (TAG) oxidation was examined with and without alpha-tocopherol. Three types of TAG from fish, linseed, and soybean oil were oxidized at 50 degrees C to determine the effect of Dihydrosphingosine (d18:0) with or without alpha-tocopherol on triacylglycerol (TAG) oxidation. The analysis of oxygen consumption and total volatile formation demonstrated a small effect of d18:0 on TAG oxidation in the absence of alpha-tocopherol. On the other hand, the combination of d18:0 with alpha-tocopherol showed strong antioxidant activity and completely inhibited volatile formation within 1400 h for soybean oil TAG, 650 h for linseed oil TAG, and 380 h for fish oil TAG.
Sphinganine potentiation of dimethyl sulfoxide-induced granulocyte differentiation, increase of alkaline phosphatase activity and decrease of protein kinase C activity in a human leukemia cell line (HL-60).[Pubmed:8135836]
Biochem Biophys Res Commun. 1994 Mar 15;199(2):888-96.
The differentiation of HL-60 promyelocytic cells toward mature granulocytic cells induced by dimethyl sulfoxide (DMSO) was accompanied by a quantitative similar increase in alkaline phosphatase (ALP) activity and decrease in protein kinase C (PKC) activity. The combination of DMSO and sphinganine (SP), a potent inhibitor of PKC, increased in parallel the percentage of mature cells and the ALP activity. The enhancement of DMSO-induced differentiation and the potentiation of the decrease of PKC activity by SP also seemed to correlate with each other. Our results indicate that both ALP and PKC may play a role in the DMSO-induced granulocytic differentiation.
Differential regulation of protein kinase C isoenzymes during sphinganine potentiation of retinoic acid-induced granulocytic differentiation in human leukemia HL-60 cells.[Pubmed:8250895]
Biochem Biophys Res Commun. 1993 Nov 15;196(3):1390-400.
Differential changes in the expression of PKC isoenzymes in the RA-induced differentiation were noted. As measured by Western blot analysis, our results indicated the expressions of PKC-alpha, and -beta isoenzymes decreased in the cell membrane but increased in the cytosol during the RA-induced granulocytic differentiation. The amounts of PKC-gamma, on the other hand, decreased in the cell membrane while there was no significant changes in the cytosol. Similarly, the expression of PKC-delta was not altered in the cytosol, but was slightly reduced during the SP enhancement of RA-induced differentiation. In contrast, there were virtually little changes in the expression of PKC-epsilon and -zeta in the cell membrane or in the cytosol during the RA-induced differentiation in the absence or presence of SP. Concomitant with the decreased total PKC activity, there was a decline in the generation of sn-1,2-diacylglycerol (DAG) during the RA-induced differentiation. SP, enhancing the RA-induced differentiation, also potentiated the decrease of DAG content.


