PRT 4165Inhibitor of Bmi1/Ring1A; blocks histone H2A ubiquitination CAS# 31083-55-3 |
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- Epidermal Growth Factor Receptor Peptide (985-996)
Catalog No.:BCC1014
CAS No.:96249-43-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 31083-55-3 | SDF | Download SDF |
| PubChem ID | 207893 | Appearance | Powder |
| Formula | C15H9NO2 | M.Wt | 235.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NSC600157 | ||
| Solubility | Soluble to 100 mM in DMSO and to 5 mM in 1eq. HCl with gentle warming | ||
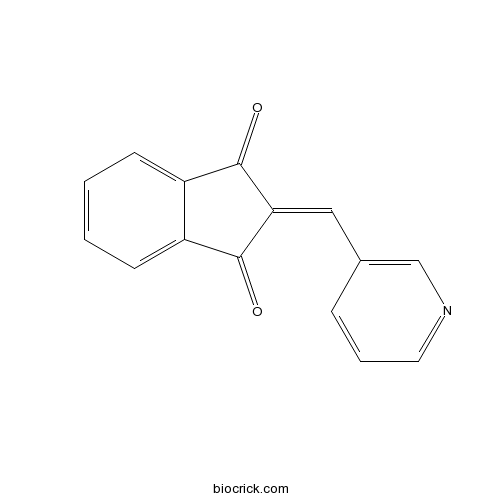
| Chemical Name | 2-(pyridin-3-ylmethylidene)indene-1,3-dione | ||
| SMILES | C1=CC=C2C(=C1)C(=O)C(=CC3=CN=CC=C3)C2=O | ||
| Standard InChIKey | OMHZFEWYVFWVLI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H9NO2/c17-14-11-5-1-2-6-12(11)15(18)13(14)8-10-4-3-7-16-9-10/h1-9H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of Bmi1/Ring1A, subunits of the polycomb repressive complex 1 (PRC1); inhibits self-ubiquitination (IC50 = 3.9 μM) but does not increase cellular levels of either subunit. Prevents Bmi1/Ring1A-mediated ubiquitination and drug-induced degradation of topoisomerase 2α (Top2α). Also shown to inhibit the in vitro E3 ubiquitin ligase activity of RNF2 and a Bmi1/RNF2 complex, inhibiting H2A/H2AX ubiquitination. Blocks polycomb repressor complex (PRC) 1-mediated histone H2A ubiquitination in vitro and in vivo. |

PRT 4165 Dilution Calculator

PRT 4165 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.251 mL | 21.2549 mL | 42.5098 mL | 85.0196 mL | 106.2744 mL |
| 5 mM | 0.8502 mL | 4.251 mL | 8.502 mL | 17.0039 mL | 21.2549 mL |
| 10 mM | 0.4251 mL | 2.1255 mL | 4.251 mL | 8.502 mL | 10.6274 mL |
| 50 mM | 0.085 mL | 0.4251 mL | 0.8502 mL | 1.7004 mL | 2.1255 mL |
| 100 mM | 0.0425 mL | 0.2125 mL | 0.4251 mL | 0.8502 mL | 1.0627 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PRT4165 is a potent inhibitor of PRC1-mediated H2A ubiquitylation.
In Vitro:PRT4165 is a potent inhibitor of PRC1-mediated H2A ubiquitylation. In vitro E3 ubiquitin ligase activity assays reveal that PRT4165 inhibits both RNF2 and RING 1A, but not RNF8 nor RNF168. In the presence of PRT4165, H2A ubiquitylation could be completely inhibited regardless of whether RING1 or RNF2 contributes the E3 ubiquitin ligase activity. Treatment of cells for 60 min with 50 μM PRT4165 results in a dramatic reduction in total ubiquitylated histone H2A. It is also found that longer exposure of the cells with the PRT4165 (30 and 60 min) leads to increased levels of γ-H2AX in unirradiated cells. PRT4165 inhibits double-strand break (DSB) repair at the 8-h time point compare with mock treated cells. Cells treated with increasing concentrations of PRT4165 show increasing numbers of cells in G2/M[1].
References:
[1]. Ismail IH, et al. A small molecule inhibitor of polycomb repressive complex 1 inhibits ubiquitin signaling at DNA double-strand breaks. J Biol Chem. 2013 Sep 13;288(37):26944-54.
- Decylic acid vanillylamide
Catalog No.:BCN7836
CAS No.:31078-36-1
- Alytesin
Catalog No.:BCC7203
CAS No.:31078-12-3
- Cedeodarin
Catalog No.:BCN4784
CAS No.:31076-39-8
- Spaglumic acid
Catalog No.:BCC6632
CAS No.:3106-85-2
- Echinophyllin C
Catalog No.:BCN5225
CAS No.:310433-44-4
- Ceramide
Catalog No.:BCC2458
CAS No.:3102-57-6
- Dihydrosphingosine
Catalog No.:BCC6778
CAS No.:3102-56-5
- Fargesin
Catalog No.:BCN5022
CAS No.:31008-19-2
- Magnolin
Catalog No.:BCN5224
CAS No.:31008-18-1
- 7-Ethoxycoumarin
Catalog No.:BCN2708
CAS No.:31005-02-4
- 1-O-(3,4-Dimethoxybenzoyl)-beta-D-glucopyranose
Catalog No.:BCN3759
CAS No.:31002-27-4
- 7-O-Methylmangiferin
Catalog No.:BCN2804
CAS No.:31002-12-7
- 5,7,3'-Trihydroxy-6,4',5'-trimethoxyflavanone
Catalog No.:BCN1461
CAS No.:310888-07-4
- Benzoquinonium dibromide
Catalog No.:BCC6641
CAS No.:311-09-1
- Taxifolin 3'-O-glucoside
Catalog No.:BCN6808
CAS No.:31106-05-5
- 3-Methyl-4-nitrobenzoic acid
Catalog No.:BCN2261
CAS No.:3113-71-1
- RFRP 3 (human)
Catalog No.:BCC6261
CAS No.:311309-27-0
- SYM 2081
Catalog No.:BCC6840
CAS No.:31137-74-3
- D-Xylose
Catalog No.:BCC8320
CAS No.:31178-70-8
- Sudan II
Catalog No.:BCN8383
CAS No.:3118-97-6
- Methylionene
Catalog No.:BCN7120
CAS No.:31197-54-3
- H-D-Ser-OH
Catalog No.:BCC2676
CAS No.:312-84-5
- Sideroxylin
Catalog No.:BCN5226
CAS No.:3122-87-0
- Eucalyptin
Catalog No.:BCN5227
CAS No.:3122-88-1
A small molecule inhibitor of polycomb repressive complex 1 inhibits ubiquitin signaling at DNA double-strand breaks.[Pubmed:23902761]
J Biol Chem. 2013 Sep 13;288(37):26944-54.
Polycomb-repressive complex 1 (PRC1)-mediated histone ubiquitylation plays an important role in aberrant gene silencing in human cancers and is a potential target for cancer therapy. Here we show that 2-pyridine-3-yl-methylene-indan-1,3-dione (PRT4165) is a potent inhibitor of PRC1-mediated H2A ubiquitylation in vivo and in vitro. The drug also inhibits the accumulation of all detectable ubiquitin at sites of DNA double-strand breaks (DSBs), the retention of several DNA damage response proteins in foci that form around DSBs, and the repair of the DSBs. In vitro E3 ubiquitin ligase activity assays revealed that PRT4165 inhibits both RNF2 and RING 1A, which are partially redundant paralogues that together account for the E3 ubiquitin ligase activity found in PRC1 complexes, but not RNF8 nor RNF168. Because ubiquitylation is completely inhibited despite the efficient recruitment of RNF8 to DSBs, our results suggest that PRC1-mediated monoubiquitylation is required for subsequent RNF8- and/or RNF168-mediated polyubiquitylation. Our results demonstrate the unique feature of PRT4165 as a novel chromatin-remodeling compound and provide a new tool for the inhibition of ubiquitylation signaling at DNA double-strand breaks.
The E3 ubiquitin-ligase Bmi1/Ring1A controls the proteasomal degradation of Top2alpha cleavage complex - a potentially new drug target.[Pubmed:19956605]
PLoS One. 2009 Dec 1;4(12):e8104.
BACKGROUND: The topoisomerases Top1, Top2alpha and Top2beta are important molecular targets for antitumor drugs, which specifically poison Top1 or Top2 isomers. While it was previously demonstrated that poisoned Top1 and Top2beta are subject to proteasomal degradation, this phenomena was not demonstrated for Top2alpha. METHODOLOGY/PRINCIPAL FINDINGS: We show here that Top2alpha is subject to drug induced proteasomal degradation as well, although at a lower rate than Top2beta. Using an siRNA screen we identified Bmi1 and Ring1A as subunits of an E3 ubiquitin ligase involved in this process. We show that silencing of Bmi1 inhibits drug-induced Top2alpha degradation, increases the persistence of Top2alpha-DNA cleavage complex, and increases Top2 drug efficacy. The Bmi1/Ring1A ligase ubiquitinates Top2alpha in-vitro and cellular overexpression of Bmi1 increases drug induced Top2alpha ubiquitination. A small-molecular weight compound, identified in a screen for inhibitors of Bmi1/Ring1A ubiquitination activity, also prevents Top2alpha ubiquitination and drug-induced Top2alpha degradation. This ubiquitination inhibitor increases the efficacy of topoisomerase 2 poisons in a synergistic manner. CONCLUSIONS/SIGNIFICANCE: The discovery that poisoned Top2alpha is undergoing proteasomal degradation combined with the involvement of Bmi1/Ring1A, allowed us to identify a small molecule that inhibits the degradation process. The Bmi1/Ring1A inhibitor sensitizes cells to Top2 drugs, suggesting that this type of drug combination will have a beneficial therapeutic outcome. As Bmi1 is also a known oncogene, elevated in numerous types of cancer, the identified Bmi1/Ring1A ubiquitin ligase inhibitors can also be potentially used to directly target the oncogenic properties of Bmi1.


