FargesinCAS# 31008-19-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 31008-19-2 | SDF | Download SDF |
| PubChem ID | 10926754 | Appearance | White powder |
| Formula | C21H22O6 | M.Wt | 370.39 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in chloroform, ethanol and methan | ||
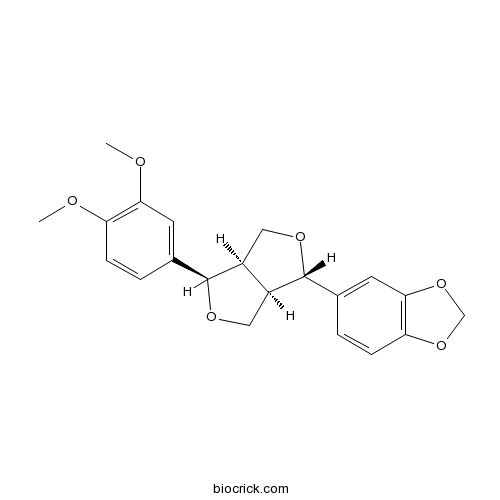
| Chemical Name | 5-[(3S,3aR,6R,6aR)-6-(3,4-dimethoxyphenyl)-1,3,3a,4,6,6a-hexahydrofuro[3,4-c]furan-3-yl]-1,3-benzodioxole | ||
| SMILES | COC1=C(C=C(C=C1)C2C3COC(C3CO2)C4=CC5=C(C=C4)OCO5)OC | ||
| Standard InChIKey | AWOGQCSIVCQXBT-LATRNWQMSA-N | ||
| Standard InChI | InChI=1S/C21H22O6/c1-22-16-5-3-12(7-18(16)23-2)20-14-9-25-21(15(14)10-24-20)13-4-6-17-19(8-13)27-11-26-17/h3-8,14-15,20-21H,9-11H2,1-2H3/t14-,15-,20-,21+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Fargesin has anti-inflammatory, anti-cancer, antihypertensive , and anti-bone-resorbing effects, it is widely used in the treatment of managing rhinitis, inflammation, histamine, sinusitis, and headache. Fargesin improves lipid and glucose metabolism in 3T3-L1 adipocytes and high-fat diet-induced obese mice by activating Akt and AMPK in WAT. It as a potential β1 adrenergic receptor antagonist protects the hearts against ischemia/reperfusion injury in rats via attenuating oxidative stress and apoptosis. |
| Targets | Akt | AMPK | GLUT | PI3K | COX | SOD | cAMP | PKA | Caspase | PPAR | TNF-α | AP-1 | NF-kB | NOS | JNK | IL Receptor | PKC | MAPK | NO | TGF-β/Smad |
| In vitro | Fargesin as a potential β1 adrenergic receptor antagonist protects the hearts against ischemia/reperfusion injury in rats via attenuating oxidative stress and apoptosis.[Pubmed: 26025856]Fitoterapia. 2015 May 27;105:16-25.Fargesin displayed similar chromatographic retention peak to metoprolol in the cardiac muscle/cell membrane chromatography (CM/CMC) and β1 adrenergic receptor/cell membrane chromatography (β1AR/CMC) models. Fargesin exerts anti-inflammatory effects in THP-1 monocytes by suppressing PKC-dependent AP-1 and NF-ĸB signaling.[Pubmed: 28160867 ]Phytomedicine. 2017 Jan 15;24:96-103.Fargesin is a lignan from Magnolia fargesii, an oriental medicine used in the treatment of nasal congestion and sinusitis. The anti-inflammatory properties of this compound have not been fully elucidated yet.
This study focused on assessing the anti-inflammatory effects of Fargesin on phorbal ester (PMA)-stimulated THP-1 human monocytes, and the molecular mechanisms underlying them.
Tetrahydrofurofuran-type lignans inhibit breast cancer-mediated bone destruction by blocking the vicious cycle between cancer cells, osteoblasts and osteoclasts.[Pubmed: 23673814]Invest New Drugs. 2014 Feb;32(1):1-13.Breast cancer frequently spreads to bone. The interaction between bone metastases and microenvironment, referred as the "vicious cycle", increases both tumor burden and bone destruction. Therefore, inhibition at any point in this "vicious cycle" can reduce malignant osteolytic lesions in patients with advanced breast cancer. |
| In vivo | Antihypertensive effects of fargesin in vitro and in vivo via attenuating oxidative stress and promoting nitric oxide release.[Pubmed: 27409158 ]Can J Physiol Pharmacol. 2016 Aug;94(8):900-6.Fargesin, a bioactive neolignan isolated from magnolia plants, is widely used in the treatment of managing rhinitis, inflammation, histamine, sinusitis, and headache. To provide more biological information about Fargesin, we investigated the effects of Fargesin on rat aortic rings and 2-kidney, 1-clip (2K1C) hypertensive rats. |
| Kinase Assay | Fargesin, a component of Flos Magnoliae, stimulates glucose uptake in L6 myotubes.[Pubmed: 22791412]Fargesin improves lipid and glucose metabolism in 3T3-L1 adipocytes and high-fat diet-induced obese mice.[Pubmed: 22674784]Biofactors. 2012 Jul-Aug;38(4):300-8.
J Nat Med. 2013 Apr;67(2):320-6.Flos Magnoliae (FM) is a commonly used Chinese medicinal herb for symptomatic relief of allergic rhinitis, sinusitis and headache. Although several FM species have been used as substitutes or adulterants for clinical use, possible differences in their pharmacological actions have not been reported. |

Fargesin Dilution Calculator

Fargesin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6999 mL | 13.4993 mL | 26.9986 mL | 53.9971 mL | 67.4964 mL |
| 5 mM | 0.54 mL | 2.6999 mL | 5.3997 mL | 10.7994 mL | 13.4993 mL |
| 10 mM | 0.27 mL | 1.3499 mL | 2.6999 mL | 5.3997 mL | 6.7496 mL |
| 50 mM | 0.054 mL | 0.27 mL | 0.54 mL | 1.0799 mL | 1.3499 mL |
| 100 mM | 0.027 mL | 0.135 mL | 0.27 mL | 0.54 mL | 0.675 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Magnolin
Catalog No.:BCN5224
CAS No.:31008-18-1
- 7-Ethoxycoumarin
Catalog No.:BCN2708
CAS No.:31005-02-4
- 1-O-(3,4-Dimethoxybenzoyl)-beta-D-glucopyranose
Catalog No.:BCN3759
CAS No.:31002-27-4
- 7-O-Methylmangiferin
Catalog No.:BCN2804
CAS No.:31002-12-7
- 3-(Boc-Amino)piperidine
Catalog No.:BCC8590
CAS No.:309956-78-3
- KL 001
Catalog No.:BCC6262
CAS No.:309928-48-1
- Boc-Aib-OH
Catalog No.:BCC3148
CAS No.:30992-29-1
- SX 011
Catalog No.:BCC7731
CAS No.:309913-42-6
- Dauricinoline
Catalog No.:BCC8162
CAS No.:30984-80-6
- Vinyl Cinnamate
Catalog No.:BCN5041
CAS No.:3098-92-8
- GW791343 HCl
Catalog No.:BCC4974
CAS No.:309712-55-8
- 1,5-Dicaffeoylquinic acid
Catalog No.:BCN5913
CAS No.:30964-13-7
- Dihydrosphingosine
Catalog No.:BCC6778
CAS No.:3102-56-5
- Ceramide
Catalog No.:BCC2458
CAS No.:3102-57-6
- Echinophyllin C
Catalog No.:BCN5225
CAS No.:310433-44-4
- Spaglumic acid
Catalog No.:BCC6632
CAS No.:3106-85-2
- Cedeodarin
Catalog No.:BCN4784
CAS No.:31076-39-8
- Alytesin
Catalog No.:BCC7203
CAS No.:31078-12-3
- Decylic acid vanillylamide
Catalog No.:BCN7836
CAS No.:31078-36-1
- PRT 4165
Catalog No.:BCC6354
CAS No.:31083-55-3
- 5,7,3'-Trihydroxy-6,4',5'-trimethoxyflavanone
Catalog No.:BCN1461
CAS No.:310888-07-4
- Benzoquinonium dibromide
Catalog No.:BCC6641
CAS No.:311-09-1
- Taxifolin 3'-O-glucoside
Catalog No.:BCN6808
CAS No.:31106-05-5
- 3-Methyl-4-nitrobenzoic acid
Catalog No.:BCN2261
CAS No.:3113-71-1
Fargesin improves lipid and glucose metabolism in 3T3-L1 adipocytes and high-fat diet-induced obese mice.[Pubmed:22674784]
Biofactors. 2012 Jul-Aug;38(4):300-8.
This study examined the effects of Fargesin, a neolignan isolated from Magnolia plants, on obesity and insulin resistance and the possible mechanisms involved in these effects in 3T3-L1 adipocytes and high-fat diet (HFD)-induced obese mice. Fargesin promoted the glucose uptake in 3T3-L1 adipocytes. In HFD-induced obese mice, Fargesin decreased the body weight gain, white adipose tissue (WAT), and plasma triglyceride, non-esterified fatty acid and glucose levels, and improved the glucose tolerance. Fargesin increased glucose transporter 4 (GLUT4) protein expression and phosphorylation of Akt, AMP-activated protein kinase (AMPK), and acetyl-CoA carboxylase (ACC) in both 3T3-L1 adipocytes and WAT of HFD-induced obese mice. Fargesin also decreased the mRNA expression levels of fatty acid oxidation-related genes, such as peroxisome proliferator-activated receptor alpha (PPARalpha), carnitine palmitoyltransferase-1 (CPT-1), uncoupling protein-2 (UCP-2) and leptin in WAT. Taken together, the present findings suggest that Fargesin improves dyslipidemia and hyperglycemia by activating Akt and AMPK in WAT.
Tetrahydrofurofuran-type lignans inhibit breast cancer-mediated bone destruction by blocking the vicious cycle between cancer cells, osteoblasts and osteoclasts.[Pubmed:23673814]
Invest New Drugs. 2014 Feb;32(1):1-13.
Breast cancer frequently spreads to bone. The interaction between bone metastases and microenvironment, referred as the "vicious cycle", increases both tumor burden and bone destruction. Therefore, inhibition at any point in this "vicious cycle" can reduce malignant osteolytic lesions in patients with advanced breast cancer. In this study, we evaluated whether tetrahydrofurofuran-type lignans derived from Magnoliae Flos, commonly used in traditional Asian medicine to treat inflammatory diseases, could block breast cancer-mediated bone loss. Aschatin, Fargesin, lirioresinol B dimethyl ether, and magnolin at noncytotoxic concentrations suppressed mRNA expression and secretion of osteolytic factor PTHrP in MDA-MB-231 metastatic human breast cancer cells. Fargesin inhibited TGF-beta-stimulated cell viability, migration, and invasion and decreased TGF-beta-induced PTHrP production in MDA-MB-231 cells. In addition, these lignans reduced RANKL/OPG ratio in PTHrP-treated hFOB1.19 human osteoblastic cells and inhibited RANKL-mediated osteoclast differentiation in mouse bone marrow macrophages. Aschatin, Fargesin, lirioresinol B dimethyl ether, and magnolin substantially reduced bone-resorbing activity of osteoclasts by inhibiting MMP-9 and cathepsin K activities. Furthermore, orally administered Fargesin inhibited tumor growth and cancer-mediated bone destruction in mice with MDA-MB-231 cells injected into calvarial tissues. Aschatin, Fargesin, lirioresinol B dimethyl ether, and magnolin blocked initiation and progression of the "vicious cycle" between breast cancer metastases and bone microenvironment by inhibiting PTHrP production in breast cancer cells and osteoclastic bone resorption. Therefore, these tetrahydrofurofuran-type lignans have the potential to serve as beneficial agents to prevent and treat cancer-induced bone destruction in breast cancer patients.
Fargesin, a component of Flos Magnoliae, stimulates glucose uptake in L6 myotubes.[Pubmed:22791412]
J Nat Med. 2013 Apr;67(2):320-6.
Flos Magnoliae (FM) is a commonly used Chinese medicinal herb for symptomatic relief of allergic rhinitis, sinusitis and headache. Although several FM species have been used as substitutes or adulterants for clinical use, possible differences in their pharmacological actions have not been reported. To confirm the effects of FM on skeletal muscle glucose metabolism, we tested the effects of several compounds isolated from FM on glucose uptake by L6 myotubes. We found that Fargesin, a component of FM, dose-dependently stimulated glucose consumption in L6 myotubes, which was accompanied by enhanced glucose transporter (GLUT)-4 translocation to the cell surface. Fargesin-stimulated glucose uptake was blocked by wortmannin, a phosphatidylinositol-3 kinase (PI3 K) inhibitor. Fargesin stimulated Akt phosphorylation, a key component in the insulin signaling pathway, which was completely inhibited by wortmannin. Here, we demonstrated that Fargesin, a bioactive component of Flos Magnoliae, increases basal glucose uptake and GLUT4 translocation in L6 myotubes by activating the PI3 K-Akt pathway.
Fargesin exerts anti-inflammatory effects in THP-1 monocytes by suppressing PKC-dependent AP-1 and NF-kB signaling.[Pubmed:28160867]
Phytomedicine. 2017 Jan 15;24:96-103.
BACKGROUND: Fargesin is a lignan from Magnolia fargesii, an oriental medicine used in the treatment of nasal congestion and sinusitis. The anti-inflammatory properties of this compound have not been fully elucidated yet. PURPOSE: This study focused on assessing the anti-inflammatory effects of Fargesin on phorbal ester (PMA)-stimulated THP-1 human monocytes, and the molecular mechanisms underlying them. METHODS: Cell viability was evaluated by MTS assay. Protein expression levels of inflammatory mediators were analyzed by Western blotting, ELISA, Immunofluorescence assay. mRNA levels were measured by Real-time PCR. Promoter activities were elucidated by Luciferase assay. RESULTS: It was found that pre-treatment with Fargesin attenuated significantly the expression of two major inflammatory mediators, cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS). Fargesin also inhibited the production of pro-inflammation cytokines (IL-1beta, TNF-alpha) and chemokine (CCL-5). Besides, nuclear translocation of transcription factors nuclear factor-kappa B (NF-kB) and activator protein-1 (AP-1), which regulate multiple pro-inflammatory genes, was suppressed by Fargesin in a PKC-dependent manner. Furthermore, among the mitogen-activated protein kinases (MAPKs), only c-Jun N-terminal kinase (JNK) was downregulated by Fargesin in a PKC-dependent manner, and this reduction was involved in PMA-induced AP-1 and NF-kB nuclear translocation attenuation, demonstrated using a specific JNK inhibitor. CONCLUSION: Taken together, our results found that Fargesin exhibits anti-inflammation effects on THP-1 cells via suppression of PKC pathway including downstream JNK, nuclear factors AP-1 and NF-kB. These results suggest that Fargesin has anti-inflammatory properties with potential applications in drug development against inflammatory disorders.
Antihypertensive effects of fargesin in vitro and in vivo via attenuating oxidative stress and promoting nitric oxide release.[Pubmed:27409158]
Can J Physiol Pharmacol. 2016 Aug;94(8):900-6.
Fargesin, a bioactive neolignan isolated from magnolia plants, is widely used in the treatment of managing rhinitis, inflammation, histamine, sinusitis, and headache. To provide more biological information about Fargesin, we investigated the effects of Fargesin on rat aortic rings and 2-kidney, 1-clip (2K1C) hypertensive rats. In vitro, Fargesin caused concentration-dependent vasorelaxation in rat isolated aortic rings induced by KCl and norepinephrine. The effect was weakened by endothelium denudation and nitric oxide (NO) synthesis inhibition. In vivo, the evolution of systolic blood pressure (SBP) was followed by weekly measurements. Angiotensin II (Ang II) and endothelin (ET) levels, NO and nitric oxide synthase (NOS), and plasma and liver oxidative stress markers were determined at the end of the experimental period. After 5 weeks of Fargesin treatment, we found that Fargesin treatment reduced SBP, cardiac hypertrophy, and Ang II and ET levels of hypertensive rats. Increased NOS activity and NO level were observed in Fargesin-treated rats. Normalisation of plasma MDA concentrations and improvement of the antioxidant defence system in plasma and liver accompanied the antihypertensive effect of Fargesin. Taken together, these results provided substantial evidences that Fargesin has antihypertensive effect in 2K1C hypertensive rats via inhibiting oxidative stress and promoting NO release.
Fargesin as a potential beta(1) adrenergic receptor antagonist protects the hearts against ischemia/reperfusion injury in rats via attenuating oxidative stress and apoptosis.[Pubmed:26025856]
Fitoterapia. 2015 Sep;105:16-25.
Fargesin displayed similar chromatographic retention peak to metoprolol in the cardiac muscle/cell membrane chromatography (CM/CMC) and beta1 adrenergic receptor/cell membrane chromatography (beta1AR/CMC) models. To provide more biological information about Fargesin, we investigated the effects of Fargesin on isoproterenol-(ISO-) induced cells injury in the high expression beta1 adrenergic receptor/Chinese hamster ovary-S (beta1AR/CHO-S) cells and occluding the left coronary artery- (LAD-) induced myocardial ischemia/reperfusion (MI/R) injury in rats. The results in vitro showed that ISO-induced canonical cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA) levels were decreased by Fargesin in beta1AR/CHO-S cells. Fargesin attenuated the serum creatine kinase (CK), lactate dehydrogenase (LDH), and improved histopathological changes of ischemic myocardium compared with the I/R rats. Similar results were obtained with Evans Blue/TTC staining, in which Fargesin notably reduced infarct size. Moreover, compared with the I/R group, Fargesin increased COX release and the activities of some endogenous antioxidative enzymes including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), but suppressed malondialdehyde (MDA), and intracellular ROS release. Additionally, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay demonstrated Fargesin suppressed myocardial apoptosis, which may be related to inhibition of caspase-3 activity. Taken together, these results provided substantial evidences that Fargesin as a potential beta1AR antagonist through cAMP/PKA pathway could protect against myocardial ischemia/reperfusion injury in rats. The underlining mechanism may be related to inhibiting oxidative stress and myocardial apoptosis.


