1,5-Dicaffeoylquinic acidCAS# 30964-13-7 |
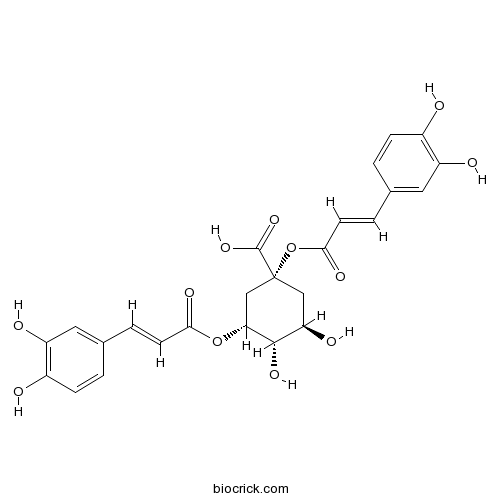
- 1,3-Dicaffeoylquinic acid
Catalog No.:BCN2972
CAS No.:19870-46-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 30964-13-7 | SDF | Download SDF |
| PubChem ID | 5281769 | Appearance | White-beige powder |
| Formula | C25H24O12 | M.Wt | 516.45 |
| Type of Compound | Phenolic Acids | Storage | Desiccate at -20°C |
| Synonyms | Cynarine | ||
| Solubility | DMSO : ≥ 23 mg/mL (44.53 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (1R,3R,4S,5R)-1,3-bis[[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy]-4,5-dihydroxycyclohexane-1-carboxylic acid | ||
| SMILES | C1C(C(C(CC1(C(=O)O)OC(=O)C=CC2=CC(=C(C=C2)O)O)OC(=O)C=CC3=CC(=C(C=C3)O)O)O)O | ||
| Standard InChIKey | YDDUMTOHNYZQPO-RVXRWRFUSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1,5-Dicaffeoylquinic acid has neuroprotective, and antioxidant effects, it can prevent Aβ(42)-induced neurotoxicity through the activation of PI3K/Akt followed by the stimulation of Trk A, then the inhibition of GSK3β as well as the modulation of Bcl-2/Bax. 1,5-Dicaffeoylquinic acid has protective effects against MPP~+ induces neurotoxicity of PC12 Cells, it (50 umol/L) pretreatment can inhibit the MPP+-induced up-regulation of the expression of α-synuclein mRNA and protein. |
| Targets | ERK | Trk receptor | Bcl-2/Bax | Beta Amyloid | PI3K | GSK-3 | ROS | Nrf2 |
| In vitro | Regulation of syringin, chlorogenic acid and 1,5-dicaffeoylquinic acid biosynthesis in cell suspension cultures of Saussurea involucrata.[Pubmed: 25244758]Zhongguo Zhong Yao Za Zhi. 2014 Jun;39(12):2275-80.Syringin, chlorogenic acid and 1,5-Dicaffeoylquinic acid are three main bioactive ingredients in herbs of Saussurea involucrata with various pharmacological properties, while their contents are very low. 1,5-Dicaffeoylquinic acid, an antioxidant component of Cynara cardunculus leaves[Reference: WebLink]Organic. Chem., 1999, 72.
1, 5-Dicaffeoylquinic acid-mediated glutathione synthesis through activation of Nrf2 protects against OGD/reperfusion-induced oxidative stress in astrocytes.[Pubmed: 20513363 ]Brain Res. 2010 Aug 6;1347:142-8.Oxidative stress plays an important role in pathological processes of cerebral ischemia followed by reperfusion. |
| Kinase Assay | 1,5-dicaffeoylquinic acid protects primary neurons from amyloid β 1-42-induced apoptosis via PI3K/Akt signaling pathway.[Pubmed: 22040415]Chin Med J (Engl). 2011 Sep;124(17):2628-35.Recently, 1,5-Dicaffeoylquinic acid (1,5-DQA), a caffeoylquinic acid derivative isolated from Aster scaber, was found to have neuroprotective effects. However, the protective mechanisms of 1,5-DQA have not yet been clearly identified. The purpose of this study was to explore the protective mechanisms of 1,5-DQA on neuronal culture.
|
| Cell Research | Protective Effects of 1,5-Dicaffeoylquinic Acid against MPP~+ Induced Neurotoxicity of PC12 Cells[Reference: WebLink]Acta Med. Universit. Sci. Et. Technol. Huazhong, 2010, 39(4): 435-38.To investigate the protective effects of 1,5-Dicaffeoylquinic acid(1,5-diCQA)preconditioning on the injury of PC12 cells induced by MPP+ and the possible mechanisms. |
| Structure Identification | Evid Based Complement Alternat Med. 2012;2012:280351.Inulae Flos and Its Compounds Inhibit TNF-α- and IFN-γ-Induced Chemokine Production in HaCaT Human Keratinocytes.[Pubmed: 22919411]
|

1,5-Dicaffeoylquinic acid Dilution Calculator

1,5-Dicaffeoylquinic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9363 mL | 9.6815 mL | 19.363 mL | 38.7259 mL | 48.4074 mL |
| 5 mM | 0.3873 mL | 1.9363 mL | 3.8726 mL | 7.7452 mL | 9.6815 mL |
| 10 mM | 0.1936 mL | 0.9681 mL | 1.9363 mL | 3.8726 mL | 4.8407 mL |
| 50 mM | 0.0387 mL | 0.1936 mL | 0.3873 mL | 0.7745 mL | 0.9681 mL |
| 100 mM | 0.0194 mL | 0.0968 mL | 0.1936 mL | 0.3873 mL | 0.4841 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cynarin is an antichoke agent with a variety of biological activities including antioxidant, antihistamic and antiviral activities.
In Vitro:Cynarin inhibits taste receptors, making water to be sweet. It has been shown to have some pharmacological properties including hypocholesterolemic, hepatoprotective, antiviral, antibacterial, and antihistamic effects. Cynarin has marked antioxidant, anticholinergic, reducing ability, radical-scavenging, and metal-binding activities. Cynarin demonstrates 87.72% inhibition of linoleic acid lipid peroxidation at 30 mg/mL concentration. Cynarin exhibits effective DMPD+, ABTS+/sup>, O2-, DPPH1, and H2O2 scavenging effects, reducing capabilities and Fe2+ chelating effects. IC50 and Ki of cynarin for acetylcholinesterase enzyme inhibition are 243.67nM and 39.34±13.88 nM, respectively[1]. Cynarin is a potential immunosuppressant that blocks the interaction between the CD28 of T-cell receptor and CD80 of antigen presenting cells. Cynarin blocks about 87% of the CD28-dependent signal 2 pathway of T-cell activation under the condition of one to one ratio of T-cell and B-cell. Cynarin binds to the G-pocket of CD28 and thus interrupts the site of interaction between CD28 and CD80[2].
References:
[1]. Topal M, et al. Antioxidant, antiradical, and anticholinergic properties of cynarin purified from the Illyrian thistle (Onopordum illyricum L.). J Enzyme Inhib Med Chem. 2016 31(2):266-75.
[2]. Dong GC, et al. Blocking effect of an immuno-suppressive agent, cynarin, on CD28 of T-cell receptor. Pharm Res. 2009 Feb 26(2):375-81.
- 2-Amino-9H-fluoren-9-one
Catalog No.:BCC8545
CAS No.:3096-57-9
- Perillartine
Catalog No.:BCN8305
CAS No.:30950-27-7
- Doxifluridine
Catalog No.:BCC4903
CAS No.:3094-09-5
- Inauhzin
Catalog No.:BCC5146
CAS No.:309271-94-1
- Boc-Asp-OBzl
Catalog No.:BCC3363
CAS No.:30925-18-9
- Boc-N-Me-Phg-OH
Catalog No.:BCC3350
CAS No.:30925-11-2
- 3β-Acetoxy-5α-androstan-17β-ol
Catalog No.:BCC8644
CAS No.:3090-70-8
- 8-Epixanthatin
Catalog No.:BCN7782
CAS No.:30890-35-8
- Kauran-18-Olc Acid,16,1719-Tnhydroxy-,(4A)
Catalog No.:BCC9235
CAS No.:308821-59-2
- Aloesin
Catalog No.:BCN8437
CAS No.:30861-27-9
- PM00104
Catalog No.:BCC4237
CAS No.:308359-57-1
- Spongouridine
Catalog No.:BCC9152
CAS No.:3083-77-0
- GW791343 HCl
Catalog No.:BCC4974
CAS No.:309712-55-8
- Vinyl Cinnamate
Catalog No.:BCN5041
CAS No.:3098-92-8
- Dauricinoline
Catalog No.:BCC8162
CAS No.:30984-80-6
- SX 011
Catalog No.:BCC7731
CAS No.:309913-42-6
- Boc-Aib-OH
Catalog No.:BCC3148
CAS No.:30992-29-1
- KL 001
Catalog No.:BCC6262
CAS No.:309928-48-1
- 3-(Boc-Amino)piperidine
Catalog No.:BCC8590
CAS No.:309956-78-3
- 7-O-Methylmangiferin
Catalog No.:BCN2804
CAS No.:31002-12-7
- 1-O-(3,4-Dimethoxybenzoyl)-beta-D-glucopyranose
Catalog No.:BCN3759
CAS No.:31002-27-4
- 7-Ethoxycoumarin
Catalog No.:BCN2708
CAS No.:31005-02-4
- Magnolin
Catalog No.:BCN5224
CAS No.:31008-18-1
- Fargesin
Catalog No.:BCN5022
CAS No.:31008-19-2
[Regulation of syringin, chlorogenic acid and 1,5-dicaffeoylquinic acid biosynthesis in cell suspension cultures of Saussurea involucrata].[Pubmed:25244758]
Zhongguo Zhong Yao Za Zhi. 2014 Jun;39(12):2275-80.
Syringin, chlorogenic acid and 1,5-Dicaffeoylquinic acid are three main bioactive ingredients in herbs of Saussurea involucrata with various pharmacological properties, while their contents are very low. In this study, the biosynthesis of syringin, chlorogenic acid and 1,5-Dicaffeoylquinic acid in the cell suspension cultures of S. involucrata were regulated by feeding carbon sources and precursors, which resulted in a great increase of the contents and yields of the above three bioactive ingredients. After 16 days of fermentation, the yields of syringin, chlorogenic acid and 1,5-Dicaffeoylquinic acid reached 339.0, 225.3, 512.7 mg x L(-1), respectively. Meanwhile, their contents increased up to 67.9, 1.9, 10.6 times of wild medicinal material, respectively. The results provided a solid basis for further studies on application of cell suspension cultures of S. involucrata for large-scale production of bioactive compounds syringin, chlorogenic acid and 1,5-Dicaffeoylquinic acid.
1,5-dicaffeoylquinic acid protects primary neurons from amyloid beta 1-42-induced apoptosis via PI3K/Akt signaling pathway.[Pubmed:22040415]
Chin Med J (Engl). 2011 Sep;124(17):2628-35.
BACKGROUND: Recently, 1,5-Dicaffeoylquinic acid (1,5-DQA), a caffeoylquinic acid derivative isolated from Aster scaber, was found to have neuroprotective effects. However, the protective mechanisms of 1,5-DQA have not yet been clearly identified. The purpose of this study was to explore the protective mechanisms of 1,5-DQA on neuronal culture. METHODS: We investigated the neuroprotective effects of 1,5-DQA against amyloid beta(1-42) (Abeta(42))-induced neurotoxicity in primary neuronal culture. To evaluate the neuroprotective effects of 1,5-DQA, primary cultured cortical neurons from neonate rats were pretreated with 1,5-DQA for 2 hours and then treated with 40 micromol/L Abeta(42) for 6 hours. Cell counting kit-8, Hoechst staining and Western blotting were used for detecting the protective mechanism. Comparisons between two groups were evaluated by independent t test, and multiple comparisons were analyzed by one-way analysis of variance (ANOVA). RESULTS: 1,5-DQA treated neurons showed increased neuronal cell viability against Abeta(42) toxicity in a concentration-dependent manner, both phosphoinositide 3-kinase (PI3K)/Akt and extracellular regulated protein kinase 1/2 (Erk1/2) were activated by 1,5-DQA with stimulating their upstream tyrosine kinase A (Trk A). However, the neuroprotective effects of 1,5-DQA were blocked by LY294002, a PI3K inhibitor, but not by PD98059, an inhibitor of mitogen-activated protein kinase kinase. Furthermore, 1,5-DQA's anti-apoptotic potential was related to the enhanced inactivating phosphorylation of glycogen synthase kinase 3beta (GSK3beta) and the modulation of expression of apoptosis-related protein Bcl-2/Bax. CONCLUSION: These results suggest that 1,5-DQA prevents Abeta(42)-induced neurotoxicity through the activation of PI3K/Akt followed by the stimulation of Trk A, then the inhibition of GSK3beta as well as the modulation of Bcl-2/Bax.
1, 5-Dicaffeoylquinic acid-mediated glutathione synthesis through activation of Nrf2 protects against OGD/reperfusion-induced oxidative stress in astrocytes.[Pubmed:20513363]
Brain Res. 2010 Aug 6;1347:142-8.
Oxidative stress plays an important role in pathological processes of cerebral ischemia followed by reperfusion. The effect of 1, 5-dicaffeoylquinic acid (1, 5-diCQA) on primary culture rat cortical astrocytes induced by oxygen and glucose deprivation (OGD)/reperfusion was evaluated in this study. Appropriate concentration of 1, 5-diCQA pretreatment significantly suppressed cell death, reduced the production of reactive oxygen species, prevented glutathione (GSH) depletion, increased the activity of glutamate-cysteine ligase (GCL), and triggered Nrf2 nuclear translocation in astrocytes induced by 4h of OGD and 20 h of reperfusion. Interestingly, these protective effects were greatly attenuated in Nrf2 siRNA-transfected cells. We conclude that 1, 5-diCQA has antioxidant signaling properties that upregulate GSH synthesis by stimulating the Nrf2 pathway in astrocytes and protects them from cell death in an in vitro model of ischemia/reperfusion.


