MebendazoleCAS# 31431-39-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 31431-39-7 | SDF | Download SDF |
| PubChem ID | 4030 | Appearance | Powder |
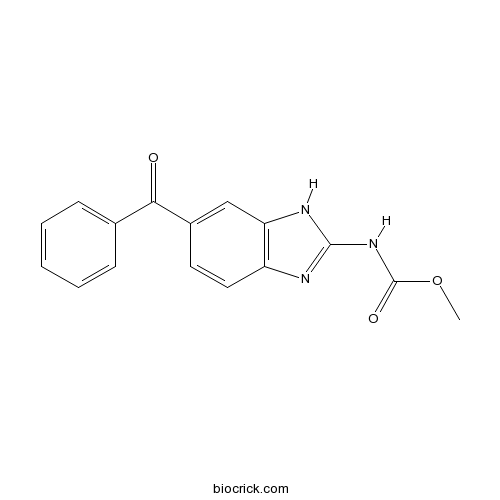
| Formula | C16H13N3O3 | M.Wt | 295.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 4.17 mg/mL (14.12 mM; Need ultrasonic) | ||
| Chemical Name | methyl N-(6-benzoyl-1H-benzimidazol-2-yl)carbamate | ||
| SMILES | COC(=O)NC1=NC2=C(N1)C=C(C=C2)C(=O)C3=CC=CC=C3 | ||
| Standard InChIKey | OPXLLQIJSORQAM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H13N3O3/c1-22-16(21)19-15-17-12-8-7-11(9-13(12)18-15)14(20)10-5-3-2-4-6-10/h2-9H,1H3,(H2,17,18,19,21) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Mebendazole Dilution Calculator

Mebendazole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3864 mL | 16.9319 mL | 33.8639 mL | 67.7277 mL | 84.6597 mL |
| 5 mM | 0.6773 mL | 3.3864 mL | 6.7728 mL | 13.5455 mL | 16.9319 mL |
| 10 mM | 0.3386 mL | 1.6932 mL | 3.3864 mL | 6.7728 mL | 8.466 mL |
| 50 mM | 0.0677 mL | 0.3386 mL | 0.6773 mL | 1.3546 mL | 1.6932 mL |
| 100 mM | 0.0339 mL | 0.1693 mL | 0.3386 mL | 0.6773 mL | 0.8466 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Mebendazole is a highly effective, broad-spectrum antihelmintic indicated for the treatment of nematode infestations; has been found as a hedgehog inhibitor.
References:
[1]. Liu C, et al. In vivo and in vitro efficacies of mebendazole, mefloquine and nitazoxanide against cyst echinococcosis. Parasitol Res. 2015 Mar 15.
[2]. Pawluk SA, et al. A review of pharmacokinetic drug-drug interactions with the anthelmintic medications albendazole and mebendazole. Clin Pharmacokinet. 2015 Apr;54(4):371-83.
[3]. Speich B, et al. Efficacy and safety of albendazole plus ivermectin, albendazole plus mebendazole, albendazole plus oxantel pamoate, and mebendazole alone against Trichuris trichiura and concomitant soil-transmitted helminth infections: a four-arm, random
[4]. Larsen AR, et al. Repurposing the antihelmintic mebendazole as a hedgehog inhibitor. Mol Cancer Ther. 2015 Jan;14(1):3-13.
- 4-Amino-3-nitrobenzophenone
Catalog No.:BCC8682
CAS No.:31431-19-3
- Nocodazole
Catalog No.:BCC3826
CAS No.:31430-18-9
- Isotachioside
Catalog No.:BCN5230
CAS No.:31427-08-4
- IU1
Catalog No.:BCC2086
CAS No.:314245-33-5
- BPTES
Catalog No.:BCC6506
CAS No.:314045-39-1
- (R)-(-)-Apomorphine hydrochloride
Catalog No.:BCC7250
CAS No.:314-19-2
- Evans Blue tetrasodium salt
Catalog No.:BCC6815
CAS No.:314-13-6
- VDM 11
Catalog No.:BCC7044
CAS No.:313998-81-1
- [cPP1-7,NPY19-23,Ala31,Aib32,Gln34] - hPancreatic Polypeptide
Catalog No.:BCC5750
CAS No.:313988-89-5
- PU 02
Catalog No.:BCC6265
CAS No.:313984-77-9
- o-3M3FBS
Catalog No.:BCC7210
CAS No.:313981-55-4
- FLI-06
Catalog No.:BCC5110
CAS No.:313967-18-9
- 6-Methoxysalicylic Acid
Catalog No.:BCC8288
CAS No.:3147-64-6
- Sunifiram
Catalog No.:BCC4167
CAS No.:314728-85-3
- Crotaline
Catalog No.:BCN4983
CAS No.:315-22-0
- Allopurinol
Catalog No.:BCC3720
CAS No.:315-30-0
- Testosterone enanthate
Catalog No.:BCC9169
CAS No.:315-37-7
- Acetylheliosupine
Catalog No.:BCN1981
CAS No.:31514-30-4
- PAC-1
Catalog No.:BCC3600
CAS No.:315183-21-2
- Ifflaiamine
Catalog No.:BCN7061
CAS No.:31520-95-3
- Sutherlandin trans-p-coumarate
Catalog No.:BCN5231
CAS No.:315236-68-1
- Isobavachin
Catalog No.:BCN5232
CAS No.:31524-62-6
- 5-Hydroxyseselin
Catalog No.:BCN3428
CAS No.:31525-75-4
- O-Nornuciferine
Catalog No.:BCN7074
CAS No.:3153-55-7
Effectiveness and Tolerability of 3-Day Mebendazole Treatment of Giardia duodenalis Infection in Adults and Children: Two Prospective, Open-Label Phase IV Trials.[Pubmed:30792825]
Curr Ther Res Clin Exp. 2018 Nov 30;89:43-47.
Background: Giardia duodenalis is the most common intestinal pathogenic protozoan infection reported in humans. Both in vitro studies and 4 separate, sequential, comparative clinical trials conducted by our group in Cuba demonstrated Mebendazole activity against G. duodenalis infection in both children and adults. Objective: The 2 additional, prospective, open-label, Phase IV follow-up studies reported here were performed to further assess the effectiveness and safety profile of Mebendazole in the outpatient treatment of G. duodenalis infection. Methods: Assenting children (n=522) whose guardians gave permission and consenting adults (n=423) diagnosed with G. duodenalis infection were given Mebendazole (200 mg 3 times daily for 3 days). Medical histories and stool samples were obtained and physical/laboratory examinations were performed pretreatment then repeated on days 3, 5, and 7 after treatment completion. The evaluation of efficacy (ie, cure) was based on parasitologic response to therapy. Participants were considered cured, if no Giardia trophozoites or cysts were found in any of the 3 posttreatment fecal specimens evaluated by direct wet mounts and/or after Ritchie concentration techniques. Results: No participant refused to be enrolled and all returned for follow-up examinations. At the end of the treatment, stool samples were negative in 450 out of 522 children (86.2%) and 392 of 423 adults (92.7%). Treatment was well tolerated. In adults, the only adverse effect reported was abdominal pain (6.2%). Side effects reported in children included abdominal pain (5.6%), nausea (2.9%), and vomiting (2.3%). Reported side effects were all mild, transient, and self-limited and did not require discontinuation of treatment or additional medication. Conclusions: Mebendazole may be an effective alternative treatment of G. duodenalis infections in both children and adults.
Autophagy Is a Potential Target for Enhancing the Anti-Angiogenic Effect of Mebendazole in Endothelial Cells.[Pubmed:30642153]
Biomol Ther (Seoul). 2019 Jan 1;27(1):117-125.
Mebendazole (MBZ), a microtubule depolymerizing drug commonly used for the treatment of helminthic infections, has recently been noted as a repositioning candidate for angiogenesis inhibition and cancer therapy. However, the definite anti-angiogenic mechanism of MBZ remains unclear. In this study, we explored the inhibitory mechanism of MBZ in endothelial cells (ECs) and developed a novel strategy to improve its anti-angiogenic therapy. Treatment of ECs with MBZ led to inhibition of EC proliferation in a dose-dependent manner in several culture conditions in the presence of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) or FBS, without selectivity of growth factors, although MBZ is known to inhibit VEGF receptor 2 kinase. Furthermore, MBZ inhibited EC migration and tube formation induced by either VEGF or bFGF. However, unexpectedly, treatment of MBZ did not affect FAK and ERK1/2 phosphorylation induced by these factors. Treatment with MBZ induced shrinking of ECs and caused G2-M arrest and apoptosis with an increased Sub-G1 fraction. In addition, increased levels of nuclear fragmentation, p53 expression, and active form of caspase 3 were observed. The marked induction of autophagy by MBZ was also noted. Interestingly, inhibition of autophagy through knocking down of Beclin1 or ATG5/7, or treatment with autophagy inhibitors such as 3-methyladenine and chloroquine resulted in marked enhancement of anti-proliferative and pro-apoptotic effects of MBZ in ECs. Consequently, we suggest that MBZ induces autophagy in ECs and that protective autophagy can be a novel target for enhancing the anti-angiogenic efficacy of MBZ in cancer treatment.


