CiclopiroxCAS# 29342-05-0 |
- Golgicide A
Catalog No.:BCC4373
CAS No.:1139889-93-2
- Tipifarnib (Zarnestra)
Catalog No.:BCC2253
CAS No.:192185-72-1
- Brefeldin A
Catalog No.:BCC4387
CAS No.:20350-15-6
- BTB06584
Catalog No.:BCC5106
CAS No.:219793-45-0
- (-)-Blebbistatin
Catalog No.:BCC4375
CAS No.:856925-71-8
- Omecamtiv mecarbil
Catalog No.:BCC3710
CAS No.:873697-71-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 29342-05-0 | SDF | Download SDF |
| PubChem ID | 2749 | Appearance | Powder |
| Formula | C12H17NO2 | M.Wt | 207.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | HOE296b | ||
| Solubility | DMSO : 100 mg/mL (482.46 mM; Need ultrasonic) | ||
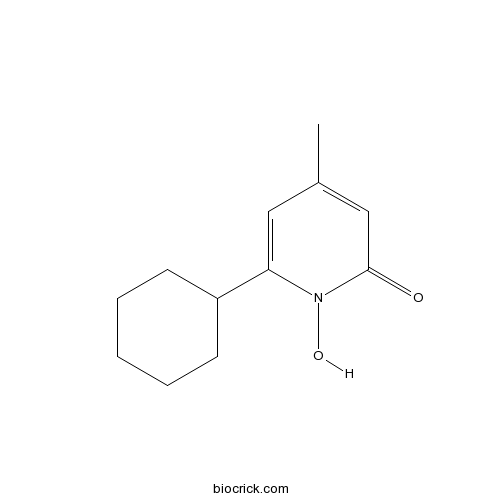
| Chemical Name | 6-cyclohexyl-1-hydroxy-4-methylpyridin-2-one | ||
| SMILES | CC1=CC(=O)N(C(=C1)C2CCCCC2)O | ||
| Standard InChIKey | SCKYRAXSEDYPSA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H17NO2/c1-9-7-11(13(15)12(14)8-9)10-5-3-2-4-6-10/h7-8,10,15H,2-6H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pan-histone demethylase inhibitor. Exhibits no significant effect on histone deacetylases. Inhibits MYC-signaling and proliferation of neuroblastoma cells. Promotes Cdc25A-degradation in cancer cells. Antifungal. |

Ciclopirox Dilution Calculator

Ciclopirox Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8246 mL | 24.1231 mL | 48.2462 mL | 96.4925 mL | 120.6156 mL |
| 5 mM | 0.9649 mL | 4.8246 mL | 9.6492 mL | 19.2985 mL | 24.1231 mL |
| 10 mM | 0.4825 mL | 2.4123 mL | 4.8246 mL | 9.6492 mL | 12.0616 mL |
| 50 mM | 0.0965 mL | 0.4825 mL | 0.9649 mL | 1.9298 mL | 2.4123 mL |
| 100 mM | 0.0482 mL | 0.2412 mL | 0.4825 mL | 0.9649 mL | 1.2062 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ciclopirox
- Tetrahydropalmatine
Catalog No.:BCN6310
CAS No.:2934-97-6
- Licarbazepine
Catalog No.:BCC7794
CAS No.:29331-92-8
- Genipin-1-O-gentiobioside
Catalog No.:BCN5349
CAS No.:29307-60-6
- Deoxyelephantopin
Catalog No.:BCN4655
CAS No.:29307-03-7
- L-685,458
Catalog No.:BCC2344
CAS No.:292632-98-5
- 25,26-Dihydroxyvitamin D3
Catalog No.:BCC4201
CAS No.:29261-12-9
- SB-3CT
Catalog No.:BCC5486
CAS No.:292605-14-2
- Dehydrotrametenolic acid
Catalog No.:BCN2718
CAS No.:29220-16-4
- L-Kynurenine
Catalog No.:BCC3899
CAS No.:2922-83-0
- H-DL-Phe(4-NO2)-OH
Catalog No.:BCC3279
CAS No.:2922-40-9
- Adenine HCl
Catalog No.:BCC4453
CAS No.:2922-28-3
- Methylprednisolone hemisuccinate
Catalog No.:BCC9044
CAS No.:2921-57-5
- Olean-12-ene-3,11-dione
Catalog No.:BCN5195
CAS No.:2935-32-2
- H-Phg-OH
Catalog No.:BCC3310
CAS No.:2935-35-5
- SR3335
Catalog No.:BCC1964
CAS No.:293753-05-6
- T0901317
Catalog No.:BCC1178
CAS No.:293754-55-9
- Thevetiaflavone
Catalog No.:BCN4024
CAS No.:29376-68-9
- Ro 90-7501
Catalog No.:BCC7351
CAS No.:293762-45-5
- Anhydrosecoisolariciresinol
Catalog No.:BCN7521
CAS No.:29388-33-8
- Secoisolariciresinol
Catalog No.:BCN5196
CAS No.:29388-59-8
- Cyclen
Catalog No.:BCN8441
CAS No.:294-90-6
- 7-Nitroindazole
Catalog No.:BCC6713
CAS No.:2942-42-9
- 4',7-Di-O-methylnaringenin
Catalog No.:BCN5197
CAS No.:29424-96-2
- Pseudolycorine
Catalog No.:BCN5371
CAS No.:29429-03-6
Antileukemia Effect of Ciclopirox Olamine Is Mediated by Downregulation of Intracellular Ferritin and Inhibition beta-Catenin-c-Myc Signaling Pathway in Glucocorticoid Resistant T-ALL Cell Lines.[Pubmed:27551974]
PLoS One. 2016 Aug 23;11(8):e0161509.
Ciclopirox olamine (CPX) is an antifungal drug that has been reported to have antitumor effects. In this study we investigated the antileukemia effects and the possible mechanisms of CPX on glucocorticoid (GC)-resistant T-cell acute lymphoblastic leukemia (T-ALL) cell lines. The results indicated that CPX inhibited the growth of GC-resistant T-ALL cells in a time- and dose-dependent manner, and this effect was closely correlated with the downregulation of intracellular ferritin. CPX induced cell cycle arrest at G1 phase by upregulation of cyclin-dependent kinase (CDK) inhibitor of p21 and downregulation of the expressions of cyclin D, retinoblastoma protein (Rb), and phosphorylated Rb (pRb). CPX also enhanced apoptotic cell death by downregulation of anti-apoptotic proteins such as Bcl-2, Bcl-xL, and Mcl-1. More importantly, CPX demonstrated a strong synergistic antileukemia effect with GC and this effect was mediated, at least in part, by inhibition of the beta-catenin-c-Myc signaling pathway. These findings suggest that CPX could be a promising antileukemia drug, and modulation of the intracellular ferritin expression might be an effective method in the treatment of ALL. Therefore, integrating CPX into the current GC-containing ALL protocols could lead to the improvement of the outcome of ALL, especially GC-resistant ALL.
Combination of ciclopirox olamine and sphingosine-1-phosphate as granulation enhancer in diabetic wounds.[Pubmed:27402256]
Wound Repair Regen. 2016 Sep;24(5):795-809.
Granulation tissue formation requires a robust angiogenic response. As granulation tissue develops, collagen fibers are deposited and compacted. Forces generated in the wake of this process drive wound contraction to reduce the wound area. In diabetics, both angiogenesis and wound contraction are diminished leading to impaired wound healing. To emulate this pathology and to address it pharmacologically, we developed a wound healing model in the diabetic Zucker fatty rat and tested a topical proangiogenic strategy combining antifungal agent Ciclopirox olamine (CPX) and lysophospholipid sphingosine-1-phosphate (S1P) to promote diabetic wound closure. In vitro, we demonstrated that CPX + S1P up-regulates a crucial driver of angiogenesis, hypoxia-inducible factor-1, in endothelial cells. Injection of CPX + S1P into subcutaneously implanted sponges in experimental rats showed, in an additive manner, a fivefold increased endothelial infiltration and lectin-perfused vessel length. We developed a splinted diabetic rodent model to achieve low wound contraction rates that are characteristic for the healing mode of diabetic ulcers in humans. We discovered specific dorsal sites that allowed for incremental full-thickness excisional wound depths from 1 mm (superficial) to 3 mm (deep). This enabled us to bring down wound contraction from 51% in superficial wounds to 8% in deep wounds. While the effects of topical gel treatment of CPX + S1P were masked by the rodent-characteristic dominant contraction in superficial wounds, they became clearly evident in deep diabetic wounds. Here, a fivefold increase of functional large vessels resulted in accelerated granulation tissue formulation, accompanied by a 40% increase of compacted thick collagen fibers. This was associated with substantially reduced matrix metalloproteinase-3 and -13 expression. These findings translated into a fivefold increase in granulation-driven contraction, promoting diabetic wound closure. With CPX and S1P analogues already in clinical use, their combination presents itself as an attractive proangiogenic treatment to be repurposed for diabetic wound healing.
Ciclopirox enhances pancreatic islet health by modulating the unfolded protein response in diabetes.[Pubmed:27757583]
Pflugers Arch. 2016 Nov;468(11-12):1957-1968.
Pancreatic dysfunction during diabetes is linked to the induction of endoplasmic reticulum (ER) stress on pancreatic beta (beta) cells. Our laboratory recently discovered that p21 protects from diabetes by modifying the outcome of ER stress response. In the present study, we explored the antidiabetic activity of Ciclopirox (CPX), an iron chelator and recently described activator of p21 expression. The effects of CPX in beta cell survival and function were assessed in cultured islets in vitro as well as in diabetic mice in vivo. The consequences of CPX in high glucose-induced insulin release and reactive oxygen species (ROS) production were also evaluated. Islet survival assays confirmed the significance of p21 in the regulation of glucotoxicity and suggested that CPX counteracts glucotoxicity in a manner that depends on p21. In vivo, administration of CPX in wild-type (WT) diabetic mice restored glucose homeostasis. In WT-cultured islets, CPX suppressed the expression of ER stress markers BiP, GRP94, and CHOP and reduced the levels of ROS during culture at high glucose. This reduction of ER stress may be associated with the ability of CPX to inhibit insulin release. Iron citrate stimulated insulin release, which was inhibited by CPX that functions as an iron chelator. It is conceivable that inhibition of insulin production constrains ER stress in islets promoting their survival and thus protecting from diabetes in vivo. This unfolded protein response (UPR)-antagonizing activity of CPX suggests application for the management not only of diabetes but also of other conditions related to ER stress.
[Clotrimazole and ciclopirox olamine respectively in combination with methylprednisolone aceponate as extemporaneous formulations].[Pubmed:28091698]
Hautarzt. 2017 Apr;68(4):307-315.
The combination of topical fungicide and glucocorticoids has been proven as a successful therapy of cutaneous mycoses with accompanying inflammatory reactions, particularly when used at an early stage. Various national and international therapeutic guidelines recommend this practice. In this context, two individually manufactured formulations have been developed and tested for stability: the combination of methylprednisolone aceponate-a topical glucocorticoid with the therapeutic index of 2.0-with clotrimazole and with Ciclopirox olamine, respectively. This has been conducted in compliance with the requirements for quality controlled extemporaneous formulations and the legal framework of the German Pharmacy Working Regulations (Apothekenbetriebsordnung). There are now two formulations for clinical use that are microbiologically, physically, and chemically stable, which combine methylprednisolone aceponate-a glucocorticoid with a good risk-benefit ratio-with the broad-spectrum fungicides clotrimazole and, for the first time, Ciclopirox olamine.


