Rhodojaponin IIICAS# 26342-66-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 26342-66-5 | SDF | Download SDF |
| PubChem ID | 3035029 | Appearance | Powder |
| Formula | C20H32O6 | M.Wt | 368.47 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
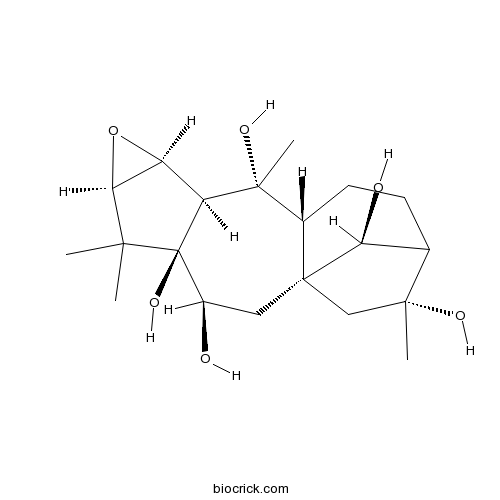
| SMILES | CC1(C2C(O2)C3C1(C(CC45CC(C(C4O)CCC5C3(C)O)(C)O)O)O)C | ||
| Standard InChIKey | VUMZHZYKXUYIHM-LISCSQOCSA-N | ||
| Standard InChI | InChI=1S/C20H32O6/c1-16(2)15-12(26-15)13-18(4,24)10-6-5-9-14(22)19(10,8-17(9,3)23)7-11(21)20(13,16)25/h9-15,21-25H,5-8H2,1-4H3/t9?,10-,11+,12-,13-,14-,15-,17+,18+,19-,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Rhodojaponin III has antifeedant and oviposition deterrence effects against many kinds of insects, BdorCSP2 of B. dorsalis could be involved in chemoreception of Rhodojaponin III and played a critical role. 2. Rhodojaponin III induces a certain linkage for change of [Ca2+](i), cell cycle arrest, proliferation inhibition in Sf9 cells. |
| Targets | Calcium Channel |

Rhodojaponin III Dilution Calculator

Rhodojaponin III Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7139 mL | 13.5696 mL | 27.1393 mL | 54.2785 mL | 67.8481 mL |
| 5 mM | 0.5428 mL | 2.7139 mL | 5.4279 mL | 10.8557 mL | 13.5696 mL |
| 10 mM | 0.2714 mL | 1.357 mL | 2.7139 mL | 5.4279 mL | 6.7848 mL |
| 50 mM | 0.0543 mL | 0.2714 mL | 0.5428 mL | 1.0856 mL | 1.357 mL |
| 100 mM | 0.0271 mL | 0.1357 mL | 0.2714 mL | 0.5428 mL | 0.6785 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- H-Arg-OMe.2HCl
Catalog No.:BCC2861
CAS No.:26340-89-6
- 1,2-Benzisothiazolin-3-one
Catalog No.:BCC8412
CAS No.:2634-33-5
- Aesculuside B
Catalog No.:BCC8115
CAS No.:26339-92-4
- Escin IB
Catalog No.:BCN2970
CAS No.:26339-90-2
- 4-(cis)-Acetyl-3,6,8-trihydroxy-3-methyldihydronaphthalenone
Catalog No.:BCN1468
CAS No.:263368-92-9
- 4-(trans)-Acetyl-3,6,8-trihydroxy-3-methyldihydronaphthalenone
Catalog No.:BCN1469
CAS No.:263368-91-8
- (S)-(-)-Pindolol
Catalog No.:BCC6916
CAS No.:26328-11-0
- Perilloxin
Catalog No.:BCN6614
CAS No.:263249-77-0
- Dehydroperilloxin
Catalog No.:BCN7506
CAS No.:263241-09-4
- 22-Dehydroclerosterol
Catalog No.:BCN5141
CAS No.:26315-07-1
- Pepstatin A
Catalog No.:BCC1218
CAS No.:26305-03-3
- Physcion-8-O-beta-D-monoglucoside
Catalog No.:BCN8511
CAS No.:26296-54-8
- H-Lys-OMe .2HCl
Catalog No.:BCC2981
CAS No.:26348-70-9
- Stachysterone D
Catalog No.:BCC8362
CAS No.:26361-67-1
- ESI-09
Catalog No.:BCC5504
CAS No.:263707-16-0
- PPT
Catalog No.:BCC7062
CAS No.:263717-53-9
- EMDT oxalate
Catalog No.:BCC7888
CAS No.:263744-72-5
- Buergerinin G
Catalog No.:BCN4659
CAS No.:263764-83-6
- 3,27-Dihydroxy-20(29)-lupen-28-oic acid methyl ester
Catalog No.:BCN1467
CAS No.:263844-79-7
- 3-Acetoxy-27-hydroxy-20(29)-lupen-28-oic acid methyl ester
Catalog No.:BCN1466
CAS No.:263844-80-0
- AG 045572
Catalog No.:BCC7464
CAS No.:263847-55-8
- S 25585
Catalog No.:BCC7687
CAS No.:263849-50-9
- DPPA (Kg)
Catalog No.:BCC2690
CAS No.:26386-88-9
- Z-Orn-OH
Catalog No.:BCC2757
CAS No.:2640-58-6
Proteomic and properties analysis of botanical insecticide rhodojaponin III-induced response of the diamondback moth, Plutella xyllostella (L.).[Pubmed:23861792]
PLoS One. 2013 Jul 5;8(7):e67723.
BACKGROUND: Rhodojaponin III, as a botanical insecticide, affects a wide variety of biological processes in insects, including reduction of feeding, suspension of development, and oviposition deterring of adults in a dose-dependent manner. However, the mode of these actions remains obscure. PRINCIPAL FINDINGS: In this study, a comparative proteomic approach was adopted to examine the effect of Rhodojaponin III on the Plutella xyllostella (L.). Following treating 48 hours, newly emergence moths were collected and protein samples were prepared. The proteins were separated by 2-DE, and total 31 proteins were significantly affected by Rhodojaponin III compared to the control identified by MALDI-TOF/TOF-MS/MS. These differentially expressed proteins act in the nervous transduction, odorant degradation and metabolic change pathways. Further, gene expression patterns in treated and untreated moths were confirmed by qRT-PCR and western blot analysis. RNAi of the chemosensory protein (PxCSP) gene resulted in oviposition significantly increased on cabbage plants treated with Rhodojaponin III. CONCLUSIONS: These Rhodojaponin III-induced proteins and gene properties analysis would be essential for a better understanding of the potential molecular mechanism of the response to Rhodojaponin III from moths of P. xylostella.
BdorCSP2 is important for antifeed and oviposition-deterring activities induced by Rhodojaponin-III against Bactrocera dorsalis.[Pubmed:24155937]
PLoS One. 2013 Oct 14;8(10):e77295.
Rhodojaponin-III is a nonvolatile botanical grayanoid diterpene compound, which has antifeedant and oviposition deterrence effects against many kinds of insects. However, the molecular mechanism of the chemoreception process remains unknown. In this study, the important role of BdorCSP2 in the recognition of Rhodojaponin-III was identified. The full length cDNA encoding BdorCSP2 was cloned from legs of Bactrocera dorsalis. The results of expression pattern revealed that BdorCSP2 was abundantly expressed in the legs of adult B. dorsalis. Moreover, the expression of BdorCSP2 could be up-regulated by Rhodojaponin-III. In order to gain comprehensive understanding of the recognition process, the binding affinity between BdorCSP2 and Rhodojaponin-III was measured by fluorescence binding assay. Silencing the expression of BdorCSP2 through the ingestion of dsRNA could weaken the effect of oviposition deterrence and antifeedant of Rhodojaponin-III. These results suggested that BdorCSP2 of B. dorsalis could be involved in chemoreception of Rhodojaponin-III and played a critical role in antifeedant and oviposition behaviors induced by Rhodojaponin-III.
Contacting is essential for oviposition deterrence of Rhodojaponin-III in Spodoptera litura.[Pubmed:24782249]
Arch Insect Biochem Physiol. 2014 Jun;86(2):122-36.
In Lepidoptera, choosing the right site for egg laying is particularly important, because the small larvae cannot forage for alternate host plants easily. Some secondary compounds of plants have the ability to deter oviposition behaviors of insects. Rhodojaponin-III, a botanical compound, has been reported to have intense deterring-oviposition activity against many insects, which have important implications for agricultural pest management. This study provided evidence for elucidating the perception mechanism underlying Rhodojaponin-III as oviposition deterrent. In this study, the antennas of moths could not elicit notable electroantennogram responses to Rhodojaponin-III, which suggested the Rhodojaponin-III could not exert effects like those volatile compounds. The results of physiological experiments confirmed the Rhodojaponin-III could produce the oviposition deterrence effect against moths without depending on antennas, while the physical contact was essential for perceiving the compound, which suggested that the sensilla on tarsus and ovipositor could be chemoreceptor for Rhodojaponin-III. Therefore, these sensilla were investigated by scanning electron microscopy to explore their potential functions in detecting Rhodojaponin-III. This study highlighted the contacting mechanism in deterring oviposition behaviors of moths by Rhodojaponin-III and provided new insight for development of contact-based pest management.
Induction of intracellular Ca2+ and pH changes in Sf9 insect cells by rhodojaponin-III, a natural botanic insecticide isolated from Rhododendron molle.[Pubmed:21499219]
Molecules. 2011 Apr 15;16(4):3179-96.
Many studies on intracellular calcium ([Ca2+](i)) and intracellular pH (pH(i)) have been carried out due to their importance in regulation of different cellular functions. However, most of the previous studies are focused on human or mammalian cells. The purpose of the present study was to characterize the effect of Rhodojaponin-III (R-III) on [Ca2+](i) and pH(i) and the proliferation of Sf9 cells. R-III strongly inhibited Sf9 cells proliferation with a time- and dose-dependent manner. Flow cytometry established that R-III interfered with Sf9 cells division and arrested them in G2/M. By using confocal scanning technique, effects of R-III on intracellular free calcium ([Ca2+](i)) and intracellular pH (pH(i)) in Sf9 cells were determined. R-III induced a significant dose-dependent (1, 10, 100, 200 mug/mL) increase in [Ca2+](i) and pH(i) of Sf9 cells in presence of Ca2+-containing solution (Hanks) and an irreversible decrease in the absence of extra cellular Ca2+. We also found that both extra cellular Ca2+ and intracellular Ca2+ stores contributed to the increase of [Ca2+](i), because completely treating Sf9 cells with CdCl(2) (5 mM), a Ca2+ channels blocker, R-III (100 mug/mL) induced a transient elevation of [Ca2+](i) in case of cells either in presence of Ca2+ containing or Ca2+ free solution. In these conditions, pH(i) showed similar changes with that of [Ca2+](i) on the whole. Accordingly, we supposed that there was a certain linkage for change of [Ca2+](i), cell cycle arrest, proliferation inhibition in Sf9 cells induced by R-III.


