S 25585Potent and selective NPY Y5 receptor antagonist CAS# 263849-50-9 |
- I-BET-762
Catalog No.:BCC4474
CAS No.:1260907-17-2
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- GSK1324726A
Catalog No.:BCC4038
CAS No.:1300031-52-0
- PFI-1 (PF-6405761)
Catalog No.:BCC2225
CAS No.:1403764-72-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 263849-50-9 | SDF | Download SDF |
| PubChem ID | 10029880 | Appearance | Powder |
| Formula | C22H23F3N4O6S | M.Wt | 528.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
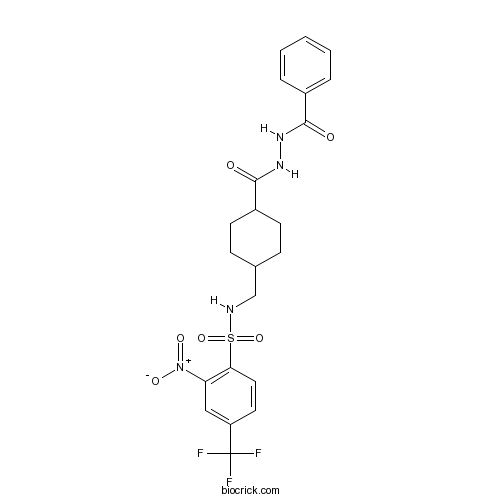
| Chemical Name | N-[[4-(benzamidocarbamoyl)cyclohexyl]methyl]-2-nitro-4-(trifluoromethyl)benzenesulfonamide | ||
| SMILES | C1CC(CCC1CNS(=O)(=O)C2=C(C=C(C=C2)C(F)(F)F)[N+](=O)[O-])C(=O)NNC(=O)C3=CC=CC=C3 | ||
| Standard InChIKey | BRMJFKLHWFZWOQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H23F3N4O6S/c23-22(24,25)17-10-11-19(18(12-17)29(32)33)36(34,35)26-13-14-6-8-16(9-7-14)21(31)28-27-20(30)15-4-2-1-3-5-15/h1-5,10-12,14,16,26H,6-9,13H2,(H,27,30)(H,28,31) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent neuropeptide Y (NPY) Y5 receptor antagonist (IC50 values are 5.4, > 1000, > 10 000 and > 10 000 nM at Y5, Y1, Y2 and Y4 receptors respectively) that displays no affinity for a wide range of other receptors. Does not produce a conditioned taste aversion, suppress sodium appetite or cause pica in rats. Significantly inhibits NPY-induced feeding but not through blockade of Y5 receptors. |

S 25585 Dilution Calculator

S 25585 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8921 mL | 9.4607 mL | 18.9215 mL | 37.843 mL | 47.3037 mL |
| 5 mM | 0.3784 mL | 1.8921 mL | 3.7843 mL | 7.5686 mL | 9.4607 mL |
| 10 mM | 0.1892 mL | 0.9461 mL | 1.8921 mL | 3.7843 mL | 4.7304 mL |
| 50 mM | 0.0378 mL | 0.1892 mL | 0.3784 mL | 0.7569 mL | 0.9461 mL |
| 100 mM | 0.0189 mL | 0.0946 mL | 0.1892 mL | 0.3784 mL | 0.473 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- AG 045572
Catalog No.:BCC7464
CAS No.:263847-55-8
- 3-Acetoxy-27-hydroxy-20(29)-lupen-28-oic acid methyl ester
Catalog No.:BCN1466
CAS No.:263844-80-0
- 3,27-Dihydroxy-20(29)-lupen-28-oic acid methyl ester
Catalog No.:BCN1467
CAS No.:263844-79-7
- Buergerinin G
Catalog No.:BCN4659
CAS No.:263764-83-6
- EMDT oxalate
Catalog No.:BCC7888
CAS No.:263744-72-5
- PPT
Catalog No.:BCC7062
CAS No.:263717-53-9
- ESI-09
Catalog No.:BCC5504
CAS No.:263707-16-0
- Stachysterone D
Catalog No.:BCC8362
CAS No.:26361-67-1
- H-Lys-OMe .2HCl
Catalog No.:BCC2981
CAS No.:26348-70-9
- Rhodojaponin III
Catalog No.:BCN2809
CAS No.:26342-66-5
- H-Arg-OMe.2HCl
Catalog No.:BCC2861
CAS No.:26340-89-6
- 1,2-Benzisothiazolin-3-one
Catalog No.:BCC8412
CAS No.:2634-33-5
- DPPA (Kg)
Catalog No.:BCC2690
CAS No.:26386-88-9
- Z-Orn-OH
Catalog No.:BCC2757
CAS No.:2640-58-6
- SB 415286
Catalog No.:BCC3651
CAS No.:264218-23-7
- Methyl 5-{2-[(tert-butylamino)carbothioyl]carbohydrazonoyl}-1-(2,4-difluorophenyl)-1H-pyrazole-4-carboxylate
Catalog No.:BCC7906
CAS No.:264233-05-8
- 6',7'-Dihydroxybergamottin
Catalog No.:BCN5142
CAS No.:264234-05-1
- Oxypeucedanin hydrate
Catalog No.:BCN2698
CAS No.:2643-85-8
- Methyl 2,2-dithienylglycolate
Catalog No.:BCC9034
CAS No.:26447-85-8
- Bz-Arg-OEt.HCl
Catalog No.:BCC2686
CAS No.:2645-08-1
- PR-619
Catalog No.:BCC3627
CAS No.:2645-32-1
- SSR 146977 hydrochloride
Catalog No.:BCC7635
CAS No.:264618-38-4
- MRS 1706
Catalog No.:BCC7120
CAS No.:264622-53-9
- MRS 1754
Catalog No.:BCC7473
CAS No.:264622-58-4
Functional characterization of human neuropeptide Y receptor subtype five specific antagonists using a luciferase reporter gene assay.[Pubmed:15601626]
Cell Signal. 2005 Apr;17(4):489-96.
Neuropeptide Y (NPY) has several receptors; one of them, the neuropeptide Y5 receptor (NPY5) seems involved in feeding behavior in mammals. Although this particular receptor has been extensively studied in the literature, the difficulties encountered to obtain a stable cell line expressing this recombinant receptor have impaired the development of tools necessary to establish its molecular pharmacology. We thus established a method for the functional study of new ligands. It is based upon the cotransfection in human melatonin receptor 1 (MT1)-overexpressing HEK293 cells of three plasmids encoding melanocortin receptor (MC5), neuropeptide Y5 receptor (NPY5) and a cyclic AMP response element-controlled luciferase. Once challenged with alphaMSH, the MC5 receptor activates the cyclic AMP response, through the coupling protein subunit G(s). In contrast, NPY5 agonists, through the NPY5 receptor which is negatively coupled to the same pathway, counteract the alphaMSH-mediated effect on cyclic AMP level. Using appropriate controls, this method can pinpoint compounds with antagonistic activity. Simple and straightforward, this system permits reproducible measurements of agonist or antagonist effects in the presence of neuropeptide Y, the natural agonist. This method has the advantage over already existing methods and beyond its apparent complexity, to enhance the cyclic AMP concentration at a 'physiological' level, by opposition to a forskolin-induced adenylate cyclase activation. Finally, to further validate this assay, we showed results from (1) a series of natural peptidic agonists that permitted the standardization and (2) a series of potent nonpeptidic antagonists (affinity >10(-9) M) that form a new class of active NPY5 receptor antagonists.


