ESI-09EPAC inhibitor, specific CAS# 263707-16-0 |
- Fulvestrant
Catalog No.:BCC1081
CAS No.:129453-61-8
- Bazedoxifene HCl
Catalog No.:BCC4492
CAS No.:198480-56-7
- XCT790
Catalog No.:BCC5121
CAS No.:725247-18-7
- Toremifene Citrate
Catalog No.:BCC4487
CAS No.:89778-27-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 263707-16-0 | SDF | Download SDF |
| PubChem ID | 6077765 | Appearance | Powder |
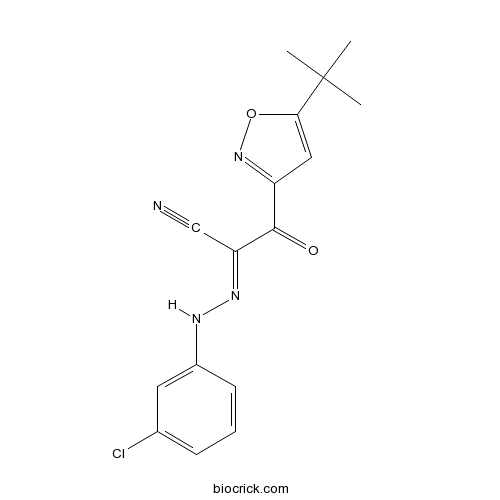
| Formula | C16H15ClN4O2 | M.Wt | 330.77 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 47 mg/mL (142.09 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (1E)-2-(5-tert-butyl-1,2-oxazol-3-yl)-N-(3-chloroanilino)-2-oxoethanimidoyl cyanide | ||
| SMILES | CC(C)(C)C1=CC(=NO1)C(=O)C(=NNC2=CC(=CC=C2)Cl)C#N | ||
| Standard InChIKey | DXEATJQGQHDURZ-DEDYPNTBSA-N | ||
| Standard InChI | InChI=1S/C16H15ClN4O2/c1-16(2,3)14-8-12(21-23-14)15(22)13(9-18)20-19-11-6-4-5-10(17)7-11/h4-8,19H,1-3H3/b20-13+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | EPAC inhibitor. Inhibits EPAC-mediated insulin releases from pancreatic β cells. Selective for EPAC over PKA. Decreases migration and invasion of AsPc-1 and PANC-1 pancreatic cancer cells in vitro. |

ESI-09 Dilution Calculator

ESI-09 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0232 mL | 15.1162 mL | 30.2325 mL | 60.465 mL | 75.5812 mL |
| 5 mM | 0.6046 mL | 3.0232 mL | 6.0465 mL | 12.093 mL | 15.1162 mL |
| 10 mM | 0.3023 mL | 1.5116 mL | 3.0232 mL | 6.0465 mL | 7.5581 mL |
| 50 mM | 0.0605 mL | 0.3023 mL | 0.6046 mL | 1.2093 mL | 1.5116 mL |
| 100 mM | 0.0302 mL | 0.1512 mL | 0.3023 mL | 0.6046 mL | 0.7558 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
ESI-09 is a specific inhibitor of EPAC with IC50 values of 1.4 and 3.2 µM for EPAC2 and EPAC1, respectively [1].
cAMP/cAMP regulated guanine nucleotide exchange factor (EPAC/cAMP-GEF) is a guanine nucleotide exchange factor for small GTPases Rap1 and Rap2 in response to intracellular cAMP [2].
ESI-09 is a specific EPAC inhibitor. ESI-09 (25 µM) reduced EPAC1 and EPAC2 GEF activity to basal levels in the presence of 25 µM cAMP. In the presence of 25 µM cAMP, ESI-09 inhibited cAMP-mediated EPAC2 and EPAC1 GEF activity with IC50 values of 1.4 and 3.2 µM respectively and exhibited 100 times selectivity than PKA. In the pancreatic cancer cell line AsPC-1, ESI-09 inhibited Akt phosphorylation at T308 and S473 stimulated by 007-AM in a dose dependent way. In pancreatic endocrine β cells, ESI-09 inhibited the increase of insulin secretion stimulated by 007-AM in a dose dependent way. In pancreatic cancer cell lines AsPC-1 and PANC-1, ESI-09 significantly reduced cell migration through the inhibition of EPAC1 [1]. In the presence of 20 µM cAMP, ESI-09 inhibited cAMP-mediated EPAC2 and EPAC1 GEF activity with IC50 values of 4.4 and 10.8 µM, respectively [2].
References:
[1]. Almahariq M, Tsalkova T, Mei FC, et al. A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol Pharmacol, 2013, 83(1): 122-128.
[2]. Zhu Y, Chen H, Boulton S, et al. Biochemical and pharmacological characterizations of ESI-09 based EPAC inhibitors: defining the ESI-09 ""therapeutic window"". Sci Rep, 2015, 5: 9344.
- Stachysterone D
Catalog No.:BCC8362
CAS No.:26361-67-1
- H-Lys-OMe .2HCl
Catalog No.:BCC2981
CAS No.:26348-70-9
- Rhodojaponin III
Catalog No.:BCN2809
CAS No.:26342-66-5
- H-Arg-OMe.2HCl
Catalog No.:BCC2861
CAS No.:26340-89-6
- 1,2-Benzisothiazolin-3-one
Catalog No.:BCC8412
CAS No.:2634-33-5
- Aesculuside B
Catalog No.:BCC8115
CAS No.:26339-92-4
- Escin IB
Catalog No.:BCN2970
CAS No.:26339-90-2
- 4-(cis)-Acetyl-3,6,8-trihydroxy-3-methyldihydronaphthalenone
Catalog No.:BCN1468
CAS No.:263368-92-9
- 4-(trans)-Acetyl-3,6,8-trihydroxy-3-methyldihydronaphthalenone
Catalog No.:BCN1469
CAS No.:263368-91-8
- (S)-(-)-Pindolol
Catalog No.:BCC6916
CAS No.:26328-11-0
- Perilloxin
Catalog No.:BCN6614
CAS No.:263249-77-0
- Dehydroperilloxin
Catalog No.:BCN7506
CAS No.:263241-09-4
- PPT
Catalog No.:BCC7062
CAS No.:263717-53-9
- EMDT oxalate
Catalog No.:BCC7888
CAS No.:263744-72-5
- Buergerinin G
Catalog No.:BCN4659
CAS No.:263764-83-6
- 3,27-Dihydroxy-20(29)-lupen-28-oic acid methyl ester
Catalog No.:BCN1467
CAS No.:263844-79-7
- 3-Acetoxy-27-hydroxy-20(29)-lupen-28-oic acid methyl ester
Catalog No.:BCN1466
CAS No.:263844-80-0
- AG 045572
Catalog No.:BCC7464
CAS No.:263847-55-8
- S 25585
Catalog No.:BCC7687
CAS No.:263849-50-9
- DPPA (Kg)
Catalog No.:BCC2690
CAS No.:26386-88-9
- Z-Orn-OH
Catalog No.:BCC2757
CAS No.:2640-58-6
- SB 415286
Catalog No.:BCC3651
CAS No.:264218-23-7
- Methyl 5-{2-[(tert-butylamino)carbothioyl]carbohydrazonoyl}-1-(2,4-difluorophenyl)-1H-pyrazole-4-carboxylate
Catalog No.:BCC7906
CAS No.:264233-05-8
- 6',7'-Dihydroxybergamottin
Catalog No.:BCN5142
CAS No.:264234-05-1
Biochemical and pharmacological characterizations of ESI-09 based EPAC inhibitors: defining the ESI-09 "therapeutic window".[Pubmed:25791905]
Sci Rep. 2015 Mar 20;5:9344.
The cAMP signaling cascade is one of the most frequently targeted pathways for the development of pharmaceutics. A plethora of recent genetic and pharmacological studies suggest that exchange proteins directly activated by cAMP (EPACs) are implicated in multiple pathologies. Selective EPAC inhibitors have been recently developed. One specific inhibitor, ESI-09, has been shown to block EPAC activity and functions, as well as to recapitulate genetic phenotypes of EPAC knockout mice when applied in vivo. However, a recent study raised concern that ESI-09 might act as a non-specific protein denaturant. Herein, we present a detailed biochemical and pharmacological characterization, as well as a structure-activity relationship (SAR) analysis of ESI-09. Our studies show that ESI-09 dose-dependently inhibits activity of both EPAC1 and EPAC2 with apparent IC50 values well below the concentrations shown to induce "protein denaturation". Moreover, the ESI-09's action towards EPAC proteins is highly sensitive to minor modifications of the 3-chlorophenyl moiety. Taken together, these results demonstrate that ESI-09 indeed acts as an EPAC specific antagonist and does not significantly destabilize/denature proteins at pharmacological effective concentrations. This conclusion is further supported by NMR data showing that ESI-09 induces residue-dependent chemical shift changes at low concentrations, while preserving well dispersed peaks.
Efficient Synthesis of ESI-09, A Novel Non-cyclic Nucleotide EPAC Antagonist.[Pubmed:23459418]
Tetrahedron Lett. 2013 Mar 20;54(12):1546-1549.
A concise and efficient synthetic approach to producing a novel non-cyclic nucleotide EPAC antagonist ESI-09 and its new analogs is reported. Key features of the synthesis include a mild and reliable one-pot procedure for an isoxazole synthon, as well as a modified one-pot protocol for the cyanomethyl ketone key intermediate. The synthesis requires inexpensive starting materials and only three linear steps for the completion in a total yield of 53%.
A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion.[Pubmed:23066090]
Mol Pharmacol. 2013 Jan;83(1):122-8.
Exchange protein directly activated by cAMP (EPAC) and cAMP-dependent protein kinase (PKA) are two intracellular receptors that mediate the effects of the prototypic second messenger cAMP. Identifying pharmacological probes for selectively modulating EPAC activity represents a significant unmet need within the research field. Herein, we report the identification and characterization of 3-(5-tert-butyl-isoxazol-3-yl)-2-[(3-chloro-phenyl)-hydrazono]-3-oxo-propionitril e (ESI-09), a novel noncyclic nucleotide EPAC antagonist that is capable of specifically blocking intracellular EPAC-mediated Rap1 activation and Akt phosphorylation, as well as EPAC-mediated insulin secretion in pancreatic beta cells. Using this novel EPAC-specific inhibitor, we have probed the functional roles of overexpression of EPAC1 in pancreatic cancer cells. Our studies show that EPAC1 plays an important role in pancreatic cancer cell migration and invasion, and thus represents a potential target for developing novel therapeutic strategies for pancreatic cancer.


