KF 38789Selective inhibitor of P-selectin-mediated cell adhesion CAS# 257292-29-8 |
- PF-4981517
Catalog No.:BCC2270
CAS No.:1390637-82-7
- Abiraterone
Catalog No.:BCC2259
CAS No.:154229-19-3
- Avasimibe
Catalog No.:BCC2274
CAS No.:166518-60-1
- Alizarin
Catalog No.:BCN3479
CAS No.:72-48-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 257292-29-8 | SDF | Download SDF |
| PubChem ID | 5373277 | Appearance | Powder |
| Formula | C19H21NO5S | M.Wt | 375.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
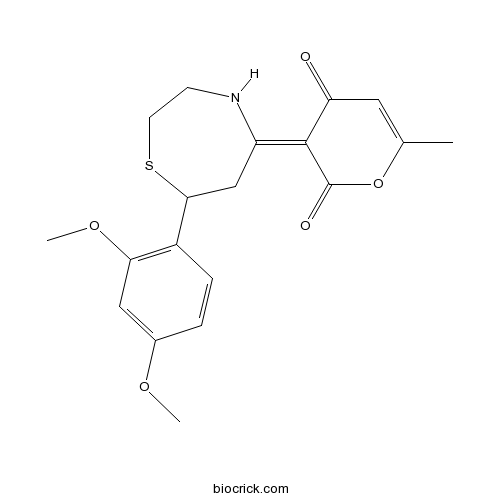
| Chemical Name | (3E)-3-[7-(2,4-dimethoxyphenyl)-1,4-thiazepan-5-ylidene]-6-methylpyran-2,4-dione | ||
| SMILES | CC1=CC(=O)C(=C2CC(SCCN2)C3=C(C=C(C=C3)OC)OC)C(=O)O1 | ||
| Standard InChIKey | FJDFKOSFDPIMBV-NBVRZTHBSA-N | ||
| Standard InChI | InChI=1S/C19H21NO5S/c1-11-8-15(21)18(19(22)25-11)14-10-17(26-7-6-20-14)13-5-4-12(23-2)9-16(13)24-3/h4-5,8-9,17,20H,6-7,10H2,1-3H3/b18-14+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective inhibitor of P-selectin-mediated cell adhesion (IC50 = 1.97 μM) that displays no effects on L-selectin- and E-selectin-mediated adhesion. Blocks P-selectin-mediated binding in vitro and leukocyte accumulation in vivo. |

KF 38789 Dilution Calculator

KF 38789 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6635 mL | 13.3177 mL | 26.6354 mL | 53.2708 mL | 66.5885 mL |
| 5 mM | 0.5327 mL | 2.6635 mL | 5.3271 mL | 10.6542 mL | 13.3177 mL |
| 10 mM | 0.2664 mL | 1.3318 mL | 2.6635 mL | 5.3271 mL | 6.6589 mL |
| 50 mM | 0.0533 mL | 0.2664 mL | 0.5327 mL | 1.0654 mL | 1.3318 mL |
| 100 mM | 0.0266 mL | 0.1332 mL | 0.2664 mL | 0.5327 mL | 0.6659 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Homoarbutin
Catalog No.:BCN2680
CAS No.:25712-94-1
- Tutin
Catalog No.:BCN5129
CAS No.:2571-22-4
- Lonicerin
Catalog No.:BCN8266
CAS No.:25694-72-8
- HU 308
Catalog No.:BCC5971
CAS No.:256934-39-1
- Cimiracemoside C
Catalog No.:BCN5128
CAS No.:256925-92-5
- CCK Octapeptide, non-sulfated
Catalog No.:BCC5709
CAS No.:25679-24-7
- 3,2'-Dihydroxy-4,4'-dimethoxychalcone
Catalog No.:BCN7742
CAS No.:2567-65-9
- Cannabigerol
Catalog No.:BCN5127
CAS No.:25654-31-3
- Aponorhyoscine
Catalog No.:BCN1871
CAS No.:25650-56-0
- Nantenine
Catalog No.:BCN7788
CAS No.:2565-01-7
- Dactylorhin A
Catalog No.:BCN8217
CAS No.:256459-34-4
- Preisocalamendiol
Catalog No.:BCN5126
CAS No.:25645-19-6
- Velutin
Catalog No.:BCN5130
CAS No.:25739-41-7
- J 104129 fumarate
Catalog No.:BCC7389
CAS No.:257603-40-0
- CP 154526
Catalog No.:BCC7481
CAS No.:257639-98-8
- H-D-Glu(OBzl)-OH
Catalog No.:BCC2939
CAS No.:2578-33-8
- Pelitinib (EKB-569)
Catalog No.:BCC1118
CAS No.:257933-82-7
- Gemfibrozil
Catalog No.:BCC4783
CAS No.:25812-30-0
- LEP (116-130) (mouse)
Catalog No.:BCC1016
CAS No.:258276-95-8
- Ghrelin (human)
Catalog No.:BCC7076
CAS No.:258279-04-8
- IEM 1925 dihydrobromide
Catalog No.:BCC7885
CAS No.:258282-23-4
- Ghrelin (rat)
Catalog No.:BCC5767
CAS No.:258338-12-4
- H-D-Cys(Trt)-OH
Catalog No.:BCC2914
CAS No.:25840-82-8
- Liensinine
Catalog No.:BCN6337
CAS No.:2586-96-1
Synthesis and biological evaluation of quinoline salicylic acids as P-selectin antagonists.[Pubmed:17201408]
J Med Chem. 2007 Jan 11;50(1):21-39.
Leukocyte recruitment of sites of inflammation and tissue injury involves leukocyte rolling along the endothelial wall, followed by firm adherence of the leukocyte, and finally transmigration of the leukocyte across cell junctions into the underlying tissue. The initial rolling step is mediated by the interaction of leukocyte glycoproteins containing active moieties such as sialyl Lewisx (sLex) with P-selectin expressed on endothelial cells. Consequently, inhibition of this interaction by means of a small molecule P-selectin antagonist is an attractive strategy for the treatment of inflammatory diseases such as arthritis. High-throughput screening of the Wyeth chemical library identified the quinoline salicylic acid class of compounds (1) as antagonists of P-selectin, with potency in in vitro and cell-based assays far superior to that of sLex. Through iterative medicinal chemistry, we identified analogues with improved P-selectin activity, decreased inhibition of dihydrooratate dehydrogenase, and acceptable CYP profiles. Lead compound 36 was efficacious in the rat AIA model of rheumatoid arthritis.
Inhibition of P-selectin specific cell adhesion by a low molecular weight, non-carbohydrate compound, KF38789.[Pubmed:11766994]
Inflamm Res. 2001 Nov;50(11):544-51.
OBJECTIVE AND DESIGN: P-selectin is a cell adhesion molecule of the selectin family. This study evaluated the effects of novel, low molecular weight P-selectin inhibitors in a cell adhesion assay and a murine model of peritonitis. MATERIALS: U937 or HL60 was used for cell adhesion assay. Human polymorphonuclear cells were studied for the production of superoxide. BALB/c mice were used for the in vivo study. TREATMENT: The thioglycollate (TG)-induced accumulation of leukocytes in mice was measured 6 h after the treatment. KF38789 or antibody (1 mg/kg) was injected intravenously prior to TG injection and at 3 h following initial injection. RESULTS: Low molecular weight, non-carbohydrate inhibitors against P-selectin- mediated cell adhesion were tested. One of the most potent inhibitors, KF38789, inhibited the binding of U937 cells to immobilized P-selectin immunoglobulin G chimeric protein (P-selectin-Ig) with an IC50 value of 1.97 microM. Cell adhesion to both E-selectin-Ig and L-selectin-Ig were not affected even by 100 microM of KF38789. Moreover, KF38789 inhibited P-selectin-induced superoxide production from human polymorphonuclear cells. Intravenously injected KF38789 significantly inhibited the TG-induced accumulation of leukocytes in the mouse peritoneal cavity (p<0.01). CONCLUSION: A novel low molecular weight compound, KF38789, specifically inhibited P-selectin-dependent cell adhesion and the leukocyte recruitment in mouse peritonitis.
Dissecting cellular processes using small molecules: identification of colchicine-like, taxol-like and other small molecules that perturb mitosis.[Pubmed:10780927]
Chem Biol. 2000 Apr;7(4):275-86.
BACKGROUND: Understanding the molecular mechanisms of complex cellular processes requires unbiased means to identify and to alter conditionally gene products that function in a pathway of interest. Although random mutagenesis and screening (forward genetics) provide a useful means to this end, the complexity of the genome, long generation time and redundancy of gene function have limited their use with mammalian systems. We sought to develop an analogous process using small molecules to modulate conditionally the function of proteins. We hoped to identify simultaneously small molecules that may serve as leads for the development of therapeutically useful agents. RESULTS: We report the results of a high-throughput, phenotype-based screen for identifying cell-permeable small molecules that affect mitosis of mammalian cells. The predominant class of compounds that emerged directly alters the stability of microtubules in the mitotic spindle. Although many of these compounds show the colchicine-like property of destabilizing microtubules, one member shows the taxol-like property of stabilizing microtubules. Another class of compounds alters chromosome segregation by novel mechanisms that do not involve direct interactions with microtubules. CONCLUSIONS: The identification of structurally diverse small molecules that affect the mammalian mitotic machinery from a large library of synthetic compounds illustrates the use of chemical genetics in dissecting an essential cellular pathway. This screen identified five compounds that affect mitosis without directly targeting microtubules. Understanding the mechanism of action of these compounds, along with future screening efforts, promises to help elucidate the molecular mechanisms involved in chromosome segregation during mitosis.


