Delta3,2-HydroxylbakuchiolCAS# 178765-49-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 178765-49-6 | SDF | Download SDF |
| PubChem ID | 15818784 | Appearance | Oil |
| Formula | C18H24O2 | M.Wt | 272.4 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
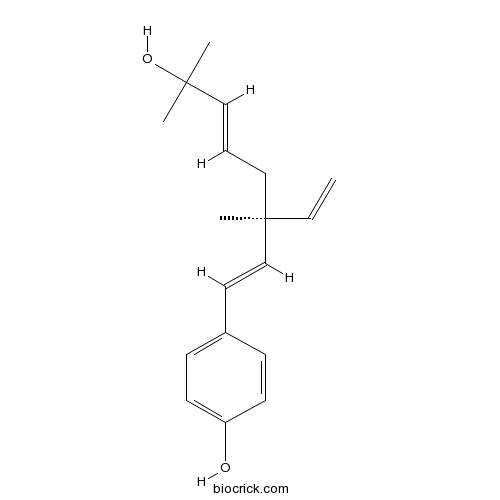
| Chemical Name | 4-[(1E,3S,5E)-3-ethenyl-7-hydroxy-3,7-dimethylocta-1,5-dienyl]phenol | ||
| SMILES | CC(C)(C=CCC(C)(C=C)C=CC1=CC=C(C=C1)O)O | ||
| Standard InChIKey | KGYDEXUROYEYFL-CEAFDCLWSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Delta3,2-Hydroxylbakuchiol acts by protecting dopaminergic neurons from MPP(+) injury and preventing against MPTP-induced behavioral and histological lesions in the Parkinson's disease (PD) model, possibly by inhibiting monoamine transporters. 2. Delta3,2-Hydroxylbakuchiol, a monoamine transporter inhibitor involved in regulating dopaminergic and noradrenergic neurotransmission and may have represented potential pharmacotherapies for disorders such as Parkinson's disease, depression, and cocaine addiction. |
| Targets | Dopamine Receptor |

Delta3,2-Hydroxylbakuchiol Dilution Calculator

Delta3,2-Hydroxylbakuchiol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6711 mL | 18.3554 mL | 36.7107 mL | 73.4214 mL | 91.7768 mL |
| 5 mM | 0.7342 mL | 3.6711 mL | 7.3421 mL | 14.6843 mL | 18.3554 mL |
| 10 mM | 0.3671 mL | 1.8355 mL | 3.6711 mL | 7.3421 mL | 9.1777 mL |
| 50 mM | 0.0734 mL | 0.3671 mL | 0.7342 mL | 1.4684 mL | 1.8355 mL |
| 100 mM | 0.0367 mL | 0.1836 mL | 0.3671 mL | 0.7342 mL | 0.9178 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Agrocybenine
Catalog No.:BCN4755
CAS No.:178764-92-6
- rel-(8R,8'R)-dimethyl-(7S,7'R)-bis(3,4-methylenedioxyphenyl)tetrahydro-furan
Catalog No.:BCN1521
CAS No.:178740-32-4
- CFM-2
Catalog No.:BCC6931
CAS No.:178616-26-7
- U-104
Catalog No.:BCC2312
CAS No.:178606-66-1
- Oleoside
Catalog No.:BCN1134
CAS No.:178600-68-5
- ZD 2079
Catalog No.:BCC5878
CAS No.:178600-17-4
- Prilocaine hydrochloride
Catalog No.:BCC4288
CAS No.:1786-81-8
- Hoechst 33342 analog
Catalog No.:BCC1630
CAS No.:178481-68-0
- Vitexin -4''-O-glucoside
Catalog No.:BCN3054
CAS No.:178468-00-3
- 6-epi-Albrassitriol
Catalog No.:BCN7342
CAS No.:178456-58-1
- Agrostophyllidin
Catalog No.:BCN3598
CAS No.:178439-50-4
- AR-R 17779 hydrochloride
Catalog No.:BCC7827
CAS No.:178419-42-6
- 3-Hydroxybakuchiol
Catalog No.:BCN3610
CAS No.:178765-54-3
- 12-Hydroxyisobakuchiol
Catalog No.:BCN3609
CAS No.:178765-55-4
- SNC 162
Catalog No.:BCC7103
CAS No.:178803-51-5
- Vitexolide D
Catalog No.:BCN6739
CAS No.:1788090-69-6
- Myricadiol
Catalog No.:BCN1135
CAS No.:17884-88-7
- Salviaflaside
Catalog No.:BCN8330
CAS No.:178895-25-5
- NGD 94-1
Catalog No.:BCC7636
CAS No.:178928-68-2
- 3-(4-Pyridyl)-Alanine
Catalog No.:BCC2651
CAS No.:178933-04-5
- FT-207 (NSC 148958)
Catalog No.:BCC4455
CAS No.:17902-23-7
- CPCCOEt
Catalog No.:BCC6896
CAS No.:179067-99-3
- PHCCC
Catalog No.:BCC6895
CAS No.:179068-02-1
- H-Asp(OtBu)-OtBu.HCl
Catalog No.:BCC2893
CAS No.:1791-13-5
Bakuchiol analogs inhibit monoamine transporters and regulate monoaminergic functions.[Pubmed:18329002]
Biochem Pharmacol. 2008 May 1;75(9):1835-47.
Monoamine transporters play key roles in controlling monoamine levels and modulating monoamine reuptake. The objective of the present study was to identify monoamine transporter inhibitors from herbal sources. We discovered that bakuchiol analogs isolated from Fructus Psoraleae inhibited monoamine transporter uptake to differing degrees. The bakuchiol analog, Delta3,2-hydroxybakuchiol was the most potent and efficacious reuptake blocker and was thus selected as the candidate target. Monoamine transporter inhibition by Delta3,2-hydroxybakuchiol was more selective for the dopamine transporter (DAT) (IC50=0.58+/-0.1 microM) and norepinephrine transporter (NET) (IC50=0.69+/-0.12 microM) than for the serotonin transporter (SERT) (IC50=312.02+/-56.69 microM). Delta3,2-Hydroxybakuchiol exhibited greater potency (pEC50 for DAT and NET) than bupropion and exhibited similar efficacy (E(max) for DAT and/or NET) to bupropion and GBR12,935. Pharmacokinetically, Delta3,2-hydroxybakuchiol competitively inhibited DAT and NET with partial reversibility and occupied cocaine binding sites. Moreover, Delta3,2-hydroxybakuchiol counteracted 1-methyl-4-phenylpyridinium-induced toxicity in cells expressing DAT with similar efficacy to GBR12,935. In vivo studies showed that Delta3,2-hydroxybakuchiol increased the activity of intact mice and improved the decreased activity of reserpinized mice. In the conditioned place preference test, preference scores in intact mice were unaffected by Delta3,2-hydroxybakuchiol treatment. Bakuchiol analogs, especially Delta3,2-hydroxybakuchiol, are monoamine transporter inhibitors involved in regulating dopaminergic and noradrenergic neurotransmission and may have represented potential pharmacotherapies for disorders such as Parkinson's disease, depression, and cocaine addiction.
In vitro dopaminergic neuroprotective and in vivo antiparkinsonian-like effects of Delta 3,2-hydroxybakuchiol isolated from Psoralea corylifolia (L.).[Pubmed:19322517]
Cell Mol Life Sci. 2009 May;66(9):1617-29.
Cocktail recipes containing Psoralea corylifolia seeds (PCS) are used to empirically treat Parkinson disease. A PCS isolate Delta(3),2-hydroxybakuchiol (BU) can inhibit dopamine uptake in dopamine transporter (DAT) transfected Chinese hamster ovary (CHO) cells, and dopamine reuptake blockade may provide an alternative approach for ameliorating parkinsonism. Here, we assessed the potential dopaminergic neuroprotective, and antiparkinsonian-like activity of BU. BU sample size was increased by using a scale-up extraction paradigm. Pharmacologically, BU significantly protected SK-N-SH cells from 1-methyl-4-phenylpyridinium (MPP(+)) insult, produced striking inhibitory actions on dopamine/norepinephrine uptake and WIN35,428 binding in synaptosomes on in vivo administration, and significantly preventing poor performance on rotarod and dopaminergic loss in substantia nigra in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mice. BU acts by protecting dopaminergic neurons from MPP(+) injury and preventing against MPTP-induced behavioral and histological lesions in the Parkinson's disease (PD) model, possibly by inhibiting monoamine transporters. These findings suggest that BU could be meaningful in PD treatment.


