Vitexolide DCAS# 1788090-69-6 |
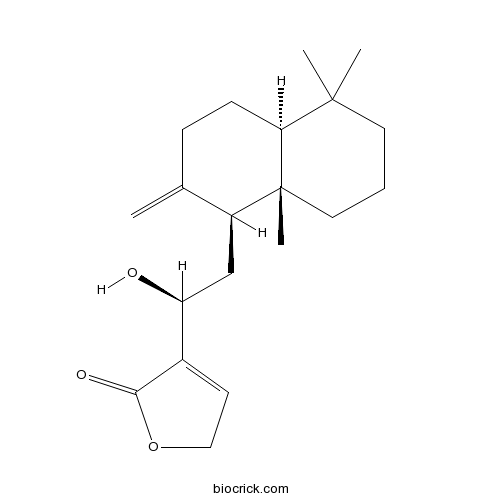
- 12-Hydroxy-8(17),13-labdadien-16,15-olide
Catalog No.:BCN1298
CAS No.:958885-86-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1788090-69-6 | SDF | Download SDF |
| PubChem ID | 16081511 | Appearance | Powder |
| Formula | C20H30O3 | M.Wt | 318.45 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-[(1S)-2-[(1S,4aS,8aS)-5,5,8a-trimethyl-2-methylidene-3,4,4a,6,7,8-hexahydro-1H-naphthalen-1-yl]-1-hydroxyethyl]-2H-furan-5-one | ||
| SMILES | CC1(CCCC2(C1CCC(=C)C2CC(C3=CCOC3=O)O)C)C | ||
| Standard InChIKey | NNNUJNWMFLYQTF-OGNFBWPZSA-N | ||
| Standard InChI | InChI=1S/C20H30O3/c1-13-6-7-17-19(2,3)9-5-10-20(17,4)15(13)12-16(21)14-8-11-23-18(14)22/h8,15-17,21H,1,5-7,9-12H2,2-4H3/t15-,16-,17-,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Vitexolide D shows moderate antibacterial activity against a panel of 46 Gram-positive strains. 2. Vitexolide D shows cytotoxic activities against the HCT-116 cancer cell line and human fetal lung fibroblast MRC5 cell line (1 < IC50s < 10 uM). |
| Targets | Antifection |

Vitexolide D Dilution Calculator

Vitexolide D Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1402 mL | 15.7011 mL | 31.4021 mL | 62.8042 mL | 78.5053 mL |
| 5 mM | 0.628 mL | 3.1402 mL | 6.2804 mL | 12.5608 mL | 15.7011 mL |
| 10 mM | 0.314 mL | 1.5701 mL | 3.1402 mL | 6.2804 mL | 7.8505 mL |
| 50 mM | 0.0628 mL | 0.314 mL | 0.628 mL | 1.2561 mL | 1.5701 mL |
| 100 mM | 0.0314 mL | 0.157 mL | 0.314 mL | 0.628 mL | 0.7851 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- SNC 162
Catalog No.:BCC7103
CAS No.:178803-51-5
- 12-Hydroxyisobakuchiol
Catalog No.:BCN3609
CAS No.:178765-55-4
- 3-Hydroxybakuchiol
Catalog No.:BCN3610
CAS No.:178765-54-3
- Delta3,2-Hydroxylbakuchiol
Catalog No.:BCN3707
CAS No.:178765-49-6
- Agrocybenine
Catalog No.:BCN4755
CAS No.:178764-92-6
- rel-(8R,8'R)-dimethyl-(7S,7'R)-bis(3,4-methylenedioxyphenyl)tetrahydro-furan
Catalog No.:BCN1521
CAS No.:178740-32-4
- CFM-2
Catalog No.:BCC6931
CAS No.:178616-26-7
- U-104
Catalog No.:BCC2312
CAS No.:178606-66-1
- Oleoside
Catalog No.:BCN1134
CAS No.:178600-68-5
- ZD 2079
Catalog No.:BCC5878
CAS No.:178600-17-4
- Prilocaine hydrochloride
Catalog No.:BCC4288
CAS No.:1786-81-8
- Hoechst 33342 analog
Catalog No.:BCC1630
CAS No.:178481-68-0
- Myricadiol
Catalog No.:BCN1135
CAS No.:17884-88-7
- Salviaflaside
Catalog No.:BCN8330
CAS No.:178895-25-5
- NGD 94-1
Catalog No.:BCC7636
CAS No.:178928-68-2
- 3-(4-Pyridyl)-Alanine
Catalog No.:BCC2651
CAS No.:178933-04-5
- FT-207 (NSC 148958)
Catalog No.:BCC4455
CAS No.:17902-23-7
- CPCCOEt
Catalog No.:BCC6896
CAS No.:179067-99-3
- PHCCC
Catalog No.:BCC6895
CAS No.:179068-02-1
- H-Asp(OtBu)-OtBu.HCl
Catalog No.:BCC2893
CAS No.:1791-13-5
- Curzerene
Catalog No.:BCN2352
CAS No.:17910-09-7
- N-Arachidonylglycine
Catalog No.:BCC7069
CAS No.:179113-91-8
- Myricitrin
Catalog No.:BCN1136
CAS No.:17912-87-7
- Zearalenone
Catalog No.:BCC7831
CAS No.:17924-92-4
Antibacterial Labdane Diterpenoids from Vitex vestita.[Pubmed:26034885]
J Nat Prod. 2015 Jun 26;78(6):1348-56.
A large-scale in vitro screening of tropical plants using an antibacterial assay permitted the selection of several species with significant antibacterial activities. Bioassay-guided purification of the dichloromethane extract of the leaves of the Malaysian species Vitex vestita, led to the isolation of six new labdane-type diterpenoids, namely, 12-epivitexolide A (2), vitexolides B and C (3 and 4), vitexolide E (8), and vitexolins A and B (5 and 6), along with six known compounds, vitexolides A (1) and D (7), acuminolide (9), 3beta-hydroxyanticopalic acid (10), 8alpha-hydroxyanticopalic acid (11), and 6alpha-hydroxyanticopalic acid (12). Their structures were elucidated on the basis of 1D and 2D NMR analyses and HRMS experiments. Both variable-temperature NMR spectroscopic studies and chemical modifications were performed to investigate the dynamic epimerization of the gamma-hydroxybutenolide moiety of compounds 1-4. Compounds were assayed against a panel of 46 Gram-positive strains. Vitexolide A (1) exhibited the most potent antibacterial activity with minimal inhibitory concentration values ranging from 6 to 96 muM, whereas compounds 2 and 6-9 showed moderate antibacterial activity. The presence of a beta-hydroxyalkyl-gamma-hydroxybutenolide subunit contributed significantly to antibacterial activity. Compounds 1-4 and 6-9 showed cytotoxic activities against the HCT-116 cancer cell line (1 < IC50s < 10 muM) and human fetal lung fibroblast MRC5 cell line (1 < IC50s < 10 muM for compounds 1, 2, 7, 8, and 9).


