ZearalenoneEstrogen receptor ligand; mycotoxin CAS# 17924-92-4 |
- ML 141
Catalog No.:BCC8092
CAS No.:71203-35-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 17924-92-4 | SDF | Download SDF |
| PubChem ID | 5281576 | Appearance | Powder |
| Formula | C18H22O5 | M.Wt | 318.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 125 mg/mL (392.64 mM; Need ultrasonic) | ||
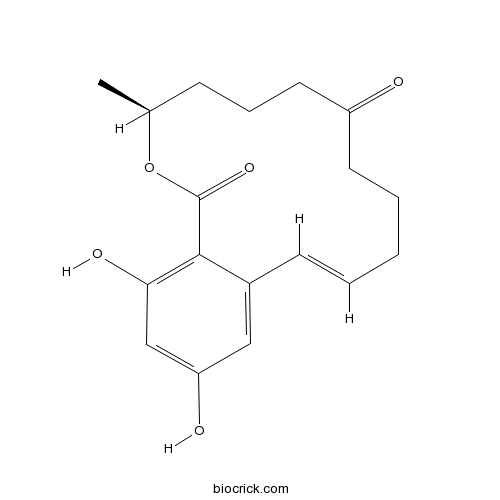
| Chemical Name | (2E,11S)-15,17-dihydroxy-11-methyl-12-oxabicyclo[12.4.0]octadeca-1(14),2,15,17-tetraene-7,13-dione | ||
| SMILES | CC1CCCC(=O)CCCC=CC2=CC(=CC(=C2C(=O)O1)O)O | ||
| Standard InChIKey | MBMQEIFVQACCCH-QBODLPLBSA-N | ||
| Standard InChI | InChI=1S/C18H22O5/c1-12-6-5-9-14(19)8-4-2-3-7-13-10-15(20)11-16(21)17(13)18(22)23-12/h3,7,10-12,20-21H,2,4-6,8-9H2,1H3/b7-3+/t12-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Mycotoxin produced by several species of Fusarium fungi. Binds to the estrogen receptor (ER) (IC50 values are 166 and 240 nM for ERβ and ERα respectively) and stimulates the transcriptional activity of both subtypes at concentrations between 1 - 10 nM. Also stimulates growth of T47D breast cancer cells. |

Zearalenone Dilution Calculator

Zearalenone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1411 mL | 15.7055 mL | 31.411 mL | 62.822 mL | 78.5275 mL |
| 5 mM | 0.6282 mL | 3.1411 mL | 6.2822 mL | 12.5644 mL | 15.7055 mL |
| 10 mM | 0.3141 mL | 1.5705 mL | 3.1411 mL | 6.2822 mL | 7.8527 mL |
| 50 mM | 0.0628 mL | 0.3141 mL | 0.6282 mL | 1.2564 mL | 1.5705 mL |
| 100 mM | 0.0314 mL | 0.1571 mL | 0.3141 mL | 0.6282 mL | 0.7853 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Myricitrin
Catalog No.:BCN1136
CAS No.:17912-87-7
- N-Arachidonylglycine
Catalog No.:BCC7069
CAS No.:179113-91-8
- Curzerene
Catalog No.:BCN2352
CAS No.:17910-09-7
- H-Asp(OtBu)-OtBu.HCl
Catalog No.:BCC2893
CAS No.:1791-13-5
- PHCCC
Catalog No.:BCC6895
CAS No.:179068-02-1
- CPCCOEt
Catalog No.:BCC6896
CAS No.:179067-99-3
- FT-207 (NSC 148958)
Catalog No.:BCC4455
CAS No.:17902-23-7
- 3-(4-Pyridyl)-Alanine
Catalog No.:BCC2651
CAS No.:178933-04-5
- NGD 94-1
Catalog No.:BCC7636
CAS No.:178928-68-2
- Salviaflaside
Catalog No.:BCN8330
CAS No.:178895-25-5
- Myricadiol
Catalog No.:BCN1135
CAS No.:17884-88-7
- Vitexolide D
Catalog No.:BCN6739
CAS No.:1788090-69-6
- Src I1
Catalog No.:BCC7733
CAS No.:179248-59-0
- Sugetriol 6,9-diacetate
Catalog No.:BCN6960
CAS No.:17928-63-1
- Bortezomib (PS-341)
Catalog No.:BCC1238
CAS No.:179324-69-7
- Eucomol
Catalog No.:BCN6820
CAS No.:17934-12-2
- 7-O-Methyleucomol
Catalog No.:BCN6830
CAS No.:17934-15-5
- Sumanirole maleate
Catalog No.:BCC4112
CAS No.:179386-44-8
- Macrocarpal H
Catalog No.:BCN1137
CAS No.:179388-53-5
- Macrocarpal I
Catalog No.:BCN1138
CAS No.:179388-54-6
- AH 11110 hydrochloride
Catalog No.:BCC6883
CAS No.:179388-65-9
- SDZ 220-581 hydrochloride
Catalog No.:BCC4157
CAS No.:179411-93-9
- SDZ 220-581 Ammonium salt
Catalog No.:BCC1940
CAS No.:179411-94-0
- 2-Methoxyanofinic acid
Catalog No.:BCN7632
CAS No.:179457-70-6
Interaction of mycotoxin zearalenone with human serum albumin.[Pubmed:28365492]
J Photochem Photobiol B. 2017 May;170:16-24.
Zearalenone (ZEN) is a mycotoxin produced mainly by Fusarium species. Fungal contamination of cereals and plants can result in the formation of ZEN, leading to its presence in different foods, animal feeds, and drinks. Because ZEN is an endocrine disruptor, it causes reproductive disorders in farm animals and hyperoestrogenic syndromes in humans. Despite toxicokinetic properties of ZEN were studied in more species, we have no information regarding the interaction of ZEN with serum albumin. Since albumin commonly plays an important role in the toxicokinetics of different toxins, interaction of ZEN with albumin has of high biological importance. Therefore the interaction of ZEN with human serum albumin (HSA) was investigated using spectroscopic methods, ultrafiltration, and molecular modeling studies. Fluorescence spectroscopic studies demonstrate that ZEN forms complex with HSA. Binding constant (K) of ZEN-HSA complex was quantified with fluorescence quenching technique. The determined binding constant (logK=5.1) reflects the strong interaction of ZEN with albumin suggesting the potential biological importance of ZEN-HSA complex formation. Based on the results of the investigations with site markers as well as docking studies, ZEN occupies a non-conventional binding site on HSA. Considering the above listed observations, we should keep in mind this interaction if we would like to precisely understand the toxicokinetic behavior of ZEN.
Adsorption of aflatoxin B1, zearalenone and ochratoxin A by microorganisms isolated from Kefir grains.[Pubmed:28376398]
Int J Food Microbiol. 2017 Jun 19;251:1-7.
A strategy to reduce the deleterious effects of mycotoxins is to use dietary supplements that contain microorganisms that bind mycotoxins and decrease their gastrointestinal absorption. Novel strains were isolated from a Kefir culture and assessed for their mycotoxin adsorption and biotransformation ability. The most active strains were identified using DNA sequencing, and the stability of microorganism/mycotoxin complexes was evaluated using buffer solutions to simulate the pH conditions in the gastrointestinal tract. Our results showed that the microorganism consortium of Kefir grains adsorbed 82 to 100% of aflatoxin B1 (AFB1), Zearalenone (ZEA) and ochratoxin A (OTA) when cultivated in milk. The main strains that were capable of mycotoxin adsorption were identified as Lactobacillus kefiri, Kazachstania servazzii and Acetobacter syzygii. The strain L. kefiri KFLM3 was the most active, adsorbing 80 to 100% of the studied mycotoxins when cultivated in milk. Nonetheless, the strain K. servazzii KFGY7 retained more mycotoxin after the desorption experiments (65, 69 and 67% for AFB1, OTA and ZEA, respectively). These findings suggest that Kefir consumption may help to reduce gastrointestinal absorption of these mycotoxins and consequently reduce their toxic effects. The isolated strains may be of interest for the development of fermented dairy products for human consumption that have a new probiotic characteristic, the adsorption of mycotoxins.
The Influence of Low Doses of Zearalenone and T-2 Toxin on Calcitonin Gene Related Peptide-Like Immunoreactive (CGRP-LI) Neurons in the ENS of the Porcine Descending Colon.[Pubmed:28287437]
Toxins (Basel). 2017 Mar 10;9(3). pii: toxins9030098.
The enteric nervous system (ENS) can undergo adaptive and reparative changes in response to physiological and pathological stimuli. These manifest primarily as alterations in the levels of active substances expressed by the enteric neuron. While it is known that mycotoxins can affect the function of the central and peripheral nervous systems, knowledge about their influence on the ENS is limited. Therefore, the aim of the present study was to investigate the influence of low doses of Zearalenone (ZEN) and T-2 toxin on calcitonin gene related peptide-like immunoreactive (CGRP-LI) neurons in the ENS of the porcine descending colon using a double immunofluorescence technique. Both mycotoxins led to an increase in the percentage of CGRP-LI neurons in all types of enteric plexuses and changed the degree of co-localization of CGRP with other neuronal active substances, such as substance P, galanin, nitric oxide synthase, and cocaine- and amphetamine-regulated transcript peptide. The obtained results demonstrate that even low doses of ZEN and T-2 can affect living organisms and cause changes in the neurochemical profile of enteric neurons.
Effects of Electron Beam Irradiation on Zearalenone and Ochratoxin A in Naturally Contaminated Corn and Corn Quality Parameters.[Pubmed:28264463]
Toxins (Basel). 2017 Feb 27;9(3). pii: toxins9030084.
Zearalenone (ZEN) and ochratoxin A (OTA) are secondary toxic metabolites widely present in grains and grain products. In this study, the effects of electron beam irradiation (EBI) on ZEN and OTA in corn and the quality of irradiated corn were investigated. Results indicated that EBI significantly affected ZEN and OTA. The degradation rates of ZEN and OTA at 10 kGy in solution were 65.6% and 75.2%, respectively. The initial amounts significantly affected the degradation rate. ZEN and OTA in corn were decreased by the irradiation dose, and their degradation rates at 50 kGy were 71.1% and 67.9%, respectively. ZEN and OTA were more easily degraded in corn kernel than in corn flour. Moisture content (MC) played a vital role in ZEN and OTA degradation. High MC was attributed to high ZEN and OTA degradation. The quality of irradiated corn was evaluated on the basis of irradiation dose. L* value changed, but this change was not significant (p > 0.05). By contrast, a* and b* decreased significantly (p < 0.05) with irradiation dose. The fatty acid value increased significantly. The pasting properties, including peak, trough, breakdown, and final and setback viscosities, were also reduced significantly (p < 0.05) by irradiation. Our study verified that EBI could effectively degrade ZEN and OTA in corn. Irradiation could also affect corn quality.
Effects of zearalenone and alpha-Zearalenol in comparison with Raloxifene on T47D cells.[Pubmed:19730705]
Toxicol Mech Methods. 2009 Mar;19(3):246-50.
Zearalenone (Zen) is a mycotoxin with estrogenic effect which contaminates cereals. In cell culture, Zen and its metabolite, alpha-Zearalenol (alpha-Zel), stimulate breast cancer cells growth. Today hormone-dependent cancers are important because of high incidence and death rate. Previous studies showed that Zen and alpha-Zel have an effect on hormone-dependent cancers. This study explains the effects of the mentioned compounds in comparison with Raloxifene as an anti-estrogen. Cell culture technique was used with MDA-MB-231 and T47D cells for evaluation of compounds. MDA-MB-231 cells were used as negative control and also for proving that treatment compounds merely affect, due to their proliferation activity in the applied doses. According to the Resazurine-based method, for toxicity assay, none of the test compounds have an effect on MDA-MB-231 cells but do effect the growth of T47D cells. Zen and alpha-Zel at low concentrations (10-8-10-9 M) stimulated T47D cell growth and Raloxifene strongly inhibited cell growth induced by Zen and alpha-Zel. There is a noticeable result in controlling diet of hormonal carcinogenic compounds and applying novel anti-estrogens for prevention and treatment of hormone-dependent cancers.
Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta.[Pubmed:9751507]
Endocrinology. 1998 Oct;139(10):4252-63.
The rat, mouse and human estrogen receptor (ER) exists as two subtypes, ER alpha and ER beta, which differ in the C-terminal ligand-binding domain and in the N-terminal transactivation domain. In this study, we investigated the estrogenic activity of environmental chemicals and phytoestrogens in competition binding assays with ER alpha or ER beta protein, and in a transient gene expression assay using cells in which an acute estrogenic response is created by cotransfecting cultures with recombinant human ER alpha or ER beta complementary DNA (cDNA) in the presence of an estrogen-dependent reporter plasmid. Saturation ligand-binding analysis of human ER alpha and ER beta protein revealed a single binding component for [3H]-17beta-estradiol (E2) with high affinity [dissociation constant (Kd) = 0.05 - 0.1 nM]. All environmental estrogenic chemicals [polychlorinated hydroxybiphenyls, dichlorodiphenyltrichloroethane (DDT) and derivatives, alkylphenols, bisphenol A, methoxychlor and chlordecone] compete with E2 for binding to both ER subtypes with a similar preference and degree. In most instances the relative binding affinities (RBA) are at least 1000-fold lower than that of E2. Some phytoestrogens such as coumestrol, genistein, apigenin, naringenin, and kaempferol compete stronger with E2 for binding to ER beta than to ER alpha. Estrogenic chemicals, as for instance nonylphenol, bisphenol A, o, p'-DDT and 2',4',6'-trichloro-4-biphenylol stimulate the transcriptional activity of ER alpha and ER beta at concentrations of 100-1000 nM. Phytoestrogens, including genistein, coumestrol and Zearalenone stimulate the transcriptional activity of both ER subtypes at concentrations of 1-10 nM. The ranking of the estrogenic potency of phytoestrogens for both ER subtypes in the transactivation assay is different; that is, E2 >> Zearalenone = coumestrol > genistein > daidzein > apigenin = phloretin > biochanin A = kaempferol = naringenin > formononetin = ipriflavone = quercetin = chrysin for ER alpha and E2 >> genistein = coumestrol > Zearalenone > daidzein > biochanin A = apigenin = kaempferol = naringenin > phloretin = quercetin = ipriflavone = formononetin = chrysin for ER beta. Antiestrogenic activity of the phytoestrogens could not be detected, except for Zearalenone which is a full agonist for ER alpha and a mixed agonist-antagonist for ER beta. In summary, while the estrogenic potency of industrial-derived estrogenic chemicals is very limited, the estrogenic potency of phytoestrogens is significant, especially for ER beta, and they may trigger many of the biological responses that are evoked by the physiological estrogens.


