BiocytinCAS# 576-19-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 576-19-2 | SDF | Download SDF |
| PubChem ID | 83814 | Appearance | Powder |
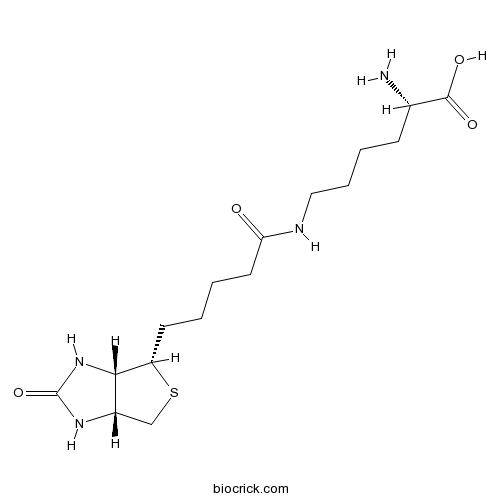
| Formula | C16H28N4O4S | M.Wt | 372.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | (+)-Biocytin | ||
| Solubility | H2O : 6 mg/mL (16.11 mM; Need ultrasonic) | ||
| Chemical Name | (2S)-6-[5-[(3aS,4S,6aR)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]pentanoylamino]-2-aminohexanoic acid | ||
| SMILES | C1C2C(C(S1)CCCCC(=O)NCCCCC(C(=O)O)N)NC(=O)N2 | ||
| Standard InChIKey | BAQMYDQNMFBZNA-MNXVOIDGSA-N | ||
| Standard InChI | InChI=1S/C16H28N4O4S/c17-10(15(22)23)5-3-4-8-18-13(21)7-2-1-6-12-14-11(9-25-12)19-16(24)20-14/h10-12,14H,1-9,17H2,(H,18,21)(H,22,23)(H2,19,20,24)/t10-,11-,12-,14-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Versatile marker used in anterograde, retrograde and intracellular neuroanatomical investigations and in biotinidase assays. Displays high solubility in aqueous solutions and has a low molecular weight facilitating injection using micropipettes. Can be incorporated with a variety of avidin and streptavidin conjugates for detection by light, fluorescence or electron microscope. |

Biocytin Dilution Calculator

Biocytin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6847 mL | 13.4235 mL | 26.8471 mL | 53.6942 mL | 67.1177 mL |
| 5 mM | 0.5369 mL | 2.6847 mL | 5.3694 mL | 10.7388 mL | 13.4235 mL |
| 10 mM | 0.2685 mL | 1.3424 mL | 2.6847 mL | 5.3694 mL | 6.7118 mL |
| 50 mM | 0.0537 mL | 0.2685 mL | 0.5369 mL | 1.0739 mL | 1.3424 mL |
| 100 mM | 0.0268 mL | 0.1342 mL | 0.2685 mL | 0.5369 mL | 0.6712 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Biocytin is a conjugate of biotin and lysine, used as a versatile marker in anterograde, retrograde and intracellular neuroanatomical investigations and in biotinidase assays.
References:
[1]. Roth G, et al. Morphology and axonal projection pattern of neurons in the telencephalon of the fire-bellied toad Bombina orientalis: an anterograde, retrograde, and intracellular biocytin labeling study. J Comp Neurol. 2004 Oct 4;478(1):35-61.
- Cycloartane-3,24,25-triol
Catalog No.:BCC8922
CAS No.:57586-98-8
- Aclacinomycin A
Catalog No.:BCC1232
CAS No.:57576-44-0
- Norcepharadione B
Catalog No.:BCN5784
CAS No.:57576-41-7
- (2,4-Dihydroxyphenyl)acetonitrile
Catalog No.:BCN5783
CAS No.:57576-34-8
- (24S)-Cycloartane-3,24,25-triol 24,25-acetonide
Catalog No.:BCN1414
CAS No.:57576-31-5
- (3beta,24xi)-Cycloartane-3,24,25-triol
Catalog No.:BCN5782
CAS No.:57576-29-1
- PNU 37883 hydrochloride
Catalog No.:BCC7262
CAS No.:57568-80-6
- Isofuranodiene
Catalog No.:BCN5781
CAS No.:57566-47-9
- SNOG
Catalog No.:BCC6714
CAS No.:57564-91-7
- Notoginsenoside S
Catalog No.:BCN8371
CAS No.:575446-95-6
- H-His(Nτ-Me)-OMe.2HCl
Catalog No.:BCC2958
CAS No.:57519-09-2
- 2-Methylsulfanylpyrimidin-4(3H)-one
Catalog No.:BCC8582
CAS No.:5751-20-2
- Piperenone
Catalog No.:BCN6578
CAS No.:57625-31-7
- Fenobam
Catalog No.:BCC7345
CAS No.:57653-26-6
- Baccatin IV
Catalog No.:BCN5785
CAS No.:57672-77-2
- 1-Dehydroxybaccatin IV
Catalog No.:BCN7211
CAS No.:57672-78-3
- Baccatin VI
Catalog No.:BCN7229
CAS No.:57672-79-4
- Palmitic acid-1-13C
Catalog No.:BCC8229
CAS No.:57677-53-9
- Kansuinine B
Catalog No.:BCN3766
CAS No.:57685-46-8
- Flavanomarein
Catalog No.:BCN6429
CAS No.:577-38-8
- 2-Acetylbenzoic acid
Catalog No.:BCN5786
CAS No.:577-56-0
- Kansuinine A
Catalog No.:BCN3765
CAS No.:57701-86-7
- CGP 7930
Catalog No.:BCC7096
CAS No.:57717-80-3
- 11,15-Dihydroxy-16-kauren-19-oic acid
Catalog No.:BCN1413
CAS No.:57719-76-3
Fast neuronal labeling in live tissue using a biocytin conjugated fluorescent probe.[Pubmed:26079494]
J Neurosci Methods. 2015 Sep 30;253:101-9.
BACKGROUND: Biocytin has found numerous uses as a neuronal tracer, since it shows both antero- and retrograde transport in neuronal tracts. The main advantage of Biocytin lies in the comprehensive intracellular distribution of the molecule, and in effective detection using avidin-based reactions. The main drawback is that Biocytin cannot be visualized in live tissue. NEW METHOD: We demonstrate that TMR Biocytin, a conjugate of Biocytin and a rhodamine fluorophore, is an effective neuronal tracer in live tissue when applied by electroporation. RESULTS: The initial fiber transport velocity of TMR Biocytin is high-5.4mm/h. TMR Biocytin can be used in conjunction with AM calcium dyes to label neuronal somas from distances of several millimetres, and record calcium transients during the course of a few hours. Juxtacellular application of TMR Biocytin leads to fast anterograde transport with labeling of local synapses within 10min. TMR Biocytin is fixable, stable during methyl salicylate clearing, and can be visualized deep in nervous tissue. COMPARISON WITH EXISTING METHODS: Retrograde labeling with TMR Biocytin enables long-range neuronal visualization and concurrent calcium imaging after only a few hours, which is substantially faster than other fluorescence-based tracers. The green emitting Atto 488 biotin is also taken up and transported retrogradely, but it is not compatible with standard green emitting calcium dyes. CONCLUSIONS: TMR Biocytin is an attractive neuronal tracer. It labels neurons fast over long distances, and it can be used in conjunction with calcium dyes to report on neuronal activity in retrogradely labeled live neurons.
Immunostaining of Biocytin-filled and Processed Sections for Neurochemical Markers.[Pubmed:28117774]
J Vis Exp. 2016 Dec 31;(118).
Electrophysiological recordings of cells using the patch clamp technique have allowed for the identification of different neuronal types based on firing patterns. The inclusion of Biocytin/neurobiotin in the recording electrode permits post-hoc recovery of morphological details, which are necessary to determine the dendritic arborization and the regions targeted by the axons of the recorded neurons. However, given the presence of morphologically similar neurons with distinct neurochemical identities and functions, immunohistochemical staining for cell-type-specific proteins is essential to definitively identify neurons. To maintain network connectivity, brain sections for physiological recordings are prepared at a thickness of 300 microm or greater. However, this thickness often hinders immunohistological postprocessing due to issues with antibody penetration, necessitating the resectioning of the tissue. Resectioning of slices is a challenging art, often resulting in the loss of tissue and morphology of the cells from which electrophysiological data was obtained, rendering the data unusable. Since recovery of morphology would limit data loss and guide in the selection of neuronal markers, we have adopted a strategy of recovering cell morphology first, followed by secondary immunostaining. We introduce a practical approach to Biocytin filling during physiological recordings and subsequent serial immunostaining for the recovery of morphology, followed by the restaining of sections to determine the neurochemical identity. We report that sections that were filled with Biocytin, fixed with paraformaldehyde (PFA), stained, and coverslipped can be removed and restained with a second primary antibody days later. This restaining involves the removal of the coverslip, the washing of sections in a buffer solution, and the incubation of primary and secondary antibodies to reveal the neurochemical identity. The method is advantageous for eliminating data loss due to an inability to recover morphology and for narrowing down the neurochemical markers to be tested based on morphology.
A comparison of manual neuronal reconstruction from biocytin histology or 2-photon imaging: morphometry and computer modeling.[Pubmed:25071470]
Front Neuroanat. 2014 Jul 11;8:65.
Accurate 3D reconstruction of neurons is vital for applications linking anatomy and physiology. Reconstructions are typically created using Neurolucida after Biocytin histology (BH). An alternative inexpensive and fast method is to use freeware such as Neuromantic to reconstruct from fluorescence imaging (FI) stacks acquired using 2-photon laser-scanning microscopy during physiological recording. We compare these two methods with respect to morphometry, cell classification, and multicompartmental modeling in the NEURON simulation environment. Quantitative morphological analysis of the same cells reconstructed using both methods reveals that whilst Biocytin reconstructions facilitate tracing of more distal collaterals, both methods are comparable in representing the overall morphology: automated clustering of reconstructions from both methods successfully separates neocortical basket cells from pyramidal cells but not BH from FI reconstructions. BH reconstructions suffer more from tissue shrinkage and compression artifacts than FI reconstructions do. FI reconstructions, on the other hand, consistently have larger process diameters. Consequently, significant differences in NEURON modeling of excitatory post-synaptic potential (EPSP) forward propagation are seen between the two methods, with FI reconstructions exhibiting smaller depolarizations. Simulated action potential backpropagation (bAP), however, is indistinguishable between reconstructions obtained with the two methods. In our hands, BH reconstructions are necessary for NEURON modeling and detailed morphological tracing, and thus remain state of the art, although they are more labor intensive, more expensive, and suffer from a higher failure rate due to the occasional poor outcome of histological processing. However, for a subset of anatomical applications such as cell type identification, FI reconstructions are superior, because of indistinguishable classification performance with greater ease of use, essentially 100% success rate, and lower cost.
Biocytin staining of glia and neurons in brain slices.[Pubmed:25183822]
Cold Spring Harb Protoc. 2014 Sep 2;2014(9):948-50.
This protocol describes the use of Biocytin to visualize and distinguish the morphology of glia and neurons in rat brain slices. Patch pipettes are used to load Biocytin into different cell types. The slices are subsequently fixed, stained, and mounted in preparation for imaging.
Biocytin wide-field bipolar cells in rabbit retina selectively contact blue cones.[Pubmed:17990268]
J Comp Neurol. 2008 Jan 1;506(1):6-15.
The Biocytin wide-field bipolar cell in rabbit retina has a broad axonal arbor in layer 5 of the inner plexiform layer and a wide dendritic arbor that does not contact all cones in its dendritic field. The purpose of our study was to identify the types of cones that this cell contacts. We identified the bipolar cells by selective uptake of Biocytin, labeled the cones with peanut agglutinin, and then used antibodies against blue cone opsin and red-green cone opsin to identify the individual cone types. The Biocytin-labeled cells selectively contacted cones whose outer segments stained for blue cone opsin and avoided cones that did not. We conclude that the Biocytin wide-field bipolar cell is an ON blue cone bipolar cell in the rabbit retina and is homologous to the blue cone bipolar cells that have been previously described in primate, mouse, and ground squirrel retinas.
Morphology and axonal projection pattern of neurons in the telencephalon of the fire-bellied toad Bombina orientalis: an anterograde, retrograde, and intracellular biocytin labeling study.[Pubmed:15334648]
J Comp Neurol. 2004 Oct 4;478(1):35-61.
The connectivity and cytoarchitecture of telencephalic centers except dorsal and medial pallium were studied in the fire-bellied toad Bombina orientalis by anterograde and retrograde Biocytin labeling and intracellular Biocytin injection (total of 148 intracellularly labeled neurons or neuron clusters). Our findings suggest the following telencephalic divisions: (1) a central amygdala-bed nucleus of the stria terminalis in the caudal midventral telencephalon, connected to visceral-autonomic centers; (2) a vomeronasal amygdala in the caudolateral ventral telencephalon receiving input from the accessory olfactory bulb and projecting mainly to the preoptic region/hypothalamus; (3) an olfactory amygdala in the caudal pole of the telencephalon lateral to the vomeronasal amygdala receiving input from the main olfactory bulb and projecting to the hypothalamus; (4) a medial amygdala receiving input from the anterior dorsal thalamus and projecting to the medial pallium, septum, and hypothalamus; (5) a ventromedial column formed by a nucleus accumbens and a ventral pallidum projecting to the central amygdala, hypothalamus, and posterior tubercle; (6) a lateral column constituting the dorsal striatum proper rostrally and the dorsal pallidum caudally, and a ventrolateral column constituting the ventral striatum. We conclude that the caudal mediolateral complex consisting of the extended central, vomeronasal, and olfactory amygdala of anurans represents the ancestral condition of the amygdaloid complex. During the evolution of the mammalian telencephalon this complex was shifted medially and involuted. The mammalian basolateral amygdala apparently is an evolutionary new structure, but the medial portion of the amygdalar complex of anurans reveals similarities in input and output with this structure and may serve similar functions.
Current concepts in neuroanatomical tracing.[Pubmed:10856608]
Prog Neurobiol. 2000 Nov;62(4):327-51.
The development of new axonal tract tracing and cell labelling methods has revolutionised neurobiology in the last 30 years. The aim of this review is to consider some of the key methods of neuroanatomical tracing that are currently in use and have proved invaluable in charting the complex interconnections of the central nervous system. The review begins with a short overview of the most frequently used tracers, including enzymes, peptides, Biocytin, latex beads, plant lectins and the ever-increasing number of fluorescent dyes. This is followed by a more detailed consideration of both well established and more recently introduced neuroanatomical tracing methods. Technical aspects of the application, uptake mechanisms, intracellular transport of tracers, and the problems of subsequent signal detection, are also discussed. The methods that are presented and discussed in detail include: (1) anterograde and retrograde neuroanatomical labelling with fluorescent dyes in vivo, (2) labelling of post mortem tissue, (3) developmental studies, (4) transcellular tracing (phagocytosis-dependent staining of glial cells), (5) electrophysiological mapping combined with neuronal tract tracing, and (6) simultaneous detection of more than one axonal tracer. (7) Versatile protocols for three-colour labelling have been developed to study complex patterns of connections. It is envisaged that this review will be used to guide the readers in their selection of the most appropriate techniques to apply to their own particular area of interest.


