AcyclovirAntiviral agent CAS# 59277-89-3 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 59277-89-3 | SDF | Download SDF |
| PubChem ID | 2022 | Appearance | Powder |
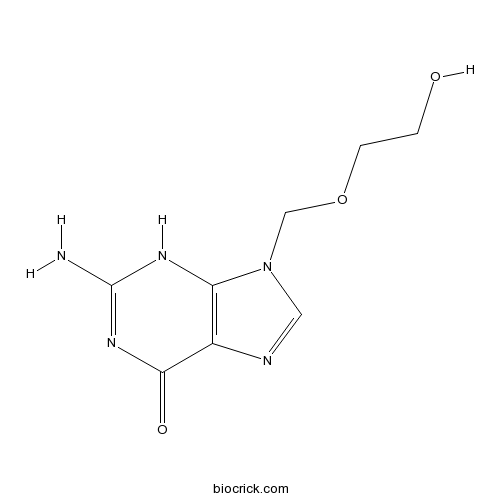
| Formula | C8H11N5O3 | M.Wt | 225.21 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Aciclovir, Acycloguanosine | ||
| Solubility | DMSO : ≥ 50 mg/mL (222.02 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-amino-9-(2-hydroxyethoxymethyl)-3H-purin-6-one | ||
| SMILES | C1=NC2=C(N1COCCO)NC(=NC2=O)N | ||
| Standard InChIKey | MKUXAQIIEYXACX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H11N5O3/c9-8-11-6-5(7(15)12-8)10-3-13(6)4-16-2-1-14/h3,14H,1-2,4H2,(H3,9,11,12,15) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Antiviral agent, active against herpes simplex viruses HSV-1 and HSV-2 (EC50 values are 0.85 and 0.86 μM respectively). Interferes with viral DNA polymerization through competitive inhibition with guanosine triphosphate. Induces apoptosis in cells transfected with HSV-TK (suicidal gene therapy). |

Acyclovir Dilution Calculator

Acyclovir Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4403 mL | 22.2015 mL | 44.403 mL | 88.806 mL | 111.0075 mL |
| 5 mM | 0.8881 mL | 4.4403 mL | 8.8806 mL | 17.7612 mL | 22.2015 mL |
| 10 mM | 0.444 mL | 2.2202 mL | 4.4403 mL | 8.8806 mL | 11.1008 mL |
| 50 mM | 0.0888 mL | 0.444 mL | 0.8881 mL | 1.7761 mL | 2.2202 mL |
| 100 mM | 0.0444 mL | 0.222 mL | 0.444 mL | 0.8881 mL | 1.1101 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Antiviral agent, active against herpes simplex viruses HSV-1 and HSV-2 (EC50 values are 0.85 and 0.86 μM respectively). Interferes with viral DNA polymerization through competitive inhibition with guanosine triphosphate. Induces apoptosis in cells transfe
- Meptazinol HCl
Catalog No.:BCC4920
CAS No.:59263-76-2
- Rigosertib sodium
Catalog No.:BCC4067
CAS No.:592542-60-4
- Rigosertib
Catalog No.:BCC4296
CAS No.:592542-59-1
- Chikusetsu Saponin Ib
Catalog No.:BCC8308
CAS No.:59252-87-8
- Laurocapram
Catalog No.:BCN8308
CAS No.:59227-89-3
- Erigeroside
Catalog No.:BCC8171
CAS No.:59219-76-0
- Darutoside
Catalog No.:BCN4094
CAS No.:59219-65-7
- 8(14),15-Isopimaradiene-3,18-diol
Catalog No.:BCN4093
CAS No.:59219-64-6
- beta-Dihydroplumericinic acid
Catalog No.:BCN4092
CAS No.:59204-61-4
- Sulforaphene
Catalog No.:BCN8179
CAS No.:592-95-0
- PSB 10 hydrochloride
Catalog No.:BCC7238
CAS No.:591771-91-4
- Neoisoliquiritin
Catalog No.:BCN2936
CAS No.:59122-93-9
- Decursin
Catalog No.:BCN5335
CAS No.:5928-25-6
- Sissotrin
Catalog No.:BCN4095
CAS No.:5928-26-7
- Vitexin 2''-O-p-coumarate
Catalog No.:BCN2793
CAS No.:59282-55-2
- Lindleyin
Catalog No.:BCN8450
CAS No.:59282-56-3
- Triacontanol
Catalog No.:BCC8260
CAS No.:593-50-0
- Alizapride HCl
Catalog No.:BCC4618
CAS No.:59338-87-3
- PIK-93
Catalog No.:BCC2519
CAS No.:593960-11-3
- Darutigenol
Catalog No.:BCN4096
CAS No.:5940-00-1
- Boc-HoSer(Bzl)-OH
Catalog No.:BCC3244
CAS No.:59408-74-1
- 1F-Fructofuranosylnystose
Catalog No.:BCN8287
CAS No.:59432-60-9
- 1-Methyl-2-undecylquinolin-4(1H)-one
Catalog No.:BCN6591
CAS No.:59443-02-6
- Monotropein
Catalog No.:BCN6280
CAS No.:5945-50-6
Association between sensitivity of viral thymidine kinase-associated acyclovir-resistant herpes simplex virus type 1 and virulence.[Pubmed:28320407]
Virol J. 2017 Mar 21;14(1):59.
BACKGROUND: Acyclovir (ACV)-resistant (ACVr) herpes simplex virus type 1 (HSV-1) infections are concern in immunocompromised patients. Most clinical ACVr HSV-1 isolates have mutations in the viral thymidine kinase (vTK) genes. The vTK-associated ACVr HSV-1 shows reduced virulence, but the association between the level of resistance and the virulence of the vTK-associated ACVr HSV-1 is still unclear. METHODS: The virulence in mice of 5 vTK-associated ACVr HSV-1 clones with a variety of ACV sensitivities, when inoculated through intracerebral and corneal routes, was evaluated in comparison with ACV-sensitive (ACVs) parent HSV-1 TAS. RESULTS: Although all the 5 ACVr HSV-1 clones and ACVs HSV-1 TAS showed a similar single-step growth capacity in vitro, the virulence of ACVr HSV-1 clones significantly decreased. A 50% lethal dose (LD50) of each clone was closely correlated with 50% inhibitory concentrations (IC50), demonstrating that the higher the ACV-sensitvity, the the higher the virulence among the ACVr clones. One of the ACVr HSV-1 clones with a relatively low IC50 value maintained similar virulence to that of the parent TAS. The infection in mice with ACVr HSV-1 due to a single amino acid substitution in vTK induced local diseases, keratitis and dermatitis, while vTK-deficient clone did not. CONCLUSIONS: A statistically significant correlation between the virulence and susceptibility to ACV among ACVr HSV-1 clones was demonstrated.
Resolution of acyclovir-associated neurotoxicity with the aid of improved clearance estimates using a Bayesian approach: A case report and review of the literature.[Pubmed:28370067]
J Clin Pharm Ther. 2017 Jun;42(3):350-355.
WHAT IS KNOWN AND OBJECTIVE: Neurotoxicity is a side effect of Acyclovir. We report the first case, to our knowledge, whereby Bayesian-informed clearance estimates supported a therapeutic intervention for Acyclovir-associated neurotoxicity. CASE SUMMARY: A 62-year-old male with the diagnosis of disseminated zoster was being treated with intravenous (IV) Acyclovir when he developed symptoms of acute neurotoxicity. Acyclovir had been dose-adjusted for renal dysfunction according to traditional creatinine clearance estimates; however, as the patient was also on vancomycin, Bayesian estimates of vancomycin clearances were performed, which revealed a 2-fold lower creatinine clearance. In response to the Bayesian estimates, Acyclovir was discontinued, and improvements in mentation were noted within 24 hours. WHAT IS NEW AND CONCLUSION: Alternate approaches to estimate renal function beyond Cockcroft-Gault, such as a Bayesian approach used in our patient, should be considered when population estimates are likely to be inaccurate and potentially dangerous to the patient.
Acyclovir-induced thrombotic microangiopathy.[Pubmed:28356666]
Indian J Nephrol. 2017 Mar-Apr;27(2):131-132.
Acyclovir is a commonly used antiviral drug. Acute kidney injury (AKI) due to intratubular crystal precipitation and interstitial nephritis is well known. Here we present a case of Acyclovir induced AKI in a 61 year old male with herpes zoster, which presented like thrombotic microangiopathy with acute interstitial nephritis. This is the first case report on Acyclovir causing thrombotic microaniopathy with partial improvement in renal function after plasmapharesis.
The role of a HSV thymidine kinase stimulating substance, scopadulciol, in improving the efficacy of cancer gene therapy.[Pubmed:16779868]
J Gene Med. 2006 Aug;8(8):1056-67.
BACKGROUND: The most extensively investigated strategy of suicide gene therapy for treatment of cancer is the transfer of the herpes simplex virus thymidine kinase (HSV-TK) gene followed by administration of antiviral prodrugs such as Acyclovir (ACV) and ganciclovir (GCV). The choice of the agent that can stimulate HSV-TK enzymatic activity is one of the determinants of the usefulness of this strategy. Previously, we found that a diterpenoid, scopadulciol (SDC), produced a significant increase in the active metabolite of ACV. This suggests that SDC may play a role in the HSV-TK/prodrug administration system. METHODS: The anticancer effect of SDC was evaluated in HSV-TK-expressing (TK+) cancer cells and nude mice bearing TK+ tumors. In vitro and in vivo enzyme assays were performed using TK+ cells and tumors. The phosphorylation of ACV monophosphate (ACV-MP) was measured in TK- cell lysates. The pharmacokinetics of prodrugs was evaluated by calculating area-under-the-concentration-time-curve values. RESULTS: SDC stimulated HSV-TK activity in TK+ cells and tumors, and increased GCV-TP levels, while no effect of SDC was observed on the phosphorylation of ACV-MP to ACV-TP by cellular kinases. The SDC/prodrug combination altered the pharmacokinetics of the prodrugs. In accord with these findings, SDC enhanced significantly the cell-killing activity of prodrugs. The bystander effect was also significantly augmented by the combined treatment of ACV/GCV and SDC. CONCLUSIONS: SDC was shown to be effective in the HSV-TK/prodrug administration system and improved the efficiency of the bystander effect of ACV and GCV. The findings will be considerably valuable with respect to the use of GCV in lower doses and less toxic ACV. This novel strategy of drug combination could provide benefit to HSV-TK/prodrug gene therapy.
Synergistic antiviral activity of acyclovir and vidarabine against herpes simplex virus types 1 and 2 and varicella-zoster virus.[Pubmed:16797734]
Antiviral Res. 2006 Nov;72(2):157-61.
Acyclovir and vidarabine both exhibit anti-herpetic activity. Because different mechanisms of action of vidarabine and Acyclovir have been reported, we analyzed their combined anti-herpetic activity on plaque formation of herpes simplex virus (HSV)-1, HSV-2, and varicella-zoster virus (VZV) by isobolograms. The results indicate that Acyclovir and vidarabine have a synergistic effect on wild type HSV-1, HSV-2, and VZV. The susceptibility of thymidine kinase-deficient HSV-1 to vidarabine was not affected by the presence of Acyclovir, suggesting that phosphorylation of Acyclovir is essential for synergism. The combined anti-HSV activity of Acyclovir and vidarabine against phosphonoacetic acid-resistant HSV-1 with DNA polymerase mutation did not show synergism in contrast to that against wild-type herpesviruses. Alteration of the substrate specificity of viral DNA polymerase to Acyclovir and vidarabine annihilated the synergism. Thus, the nature of their binding sites on DNA polymerase is important to the synergistic anti-herpesvirus activity of Acyclovir and vidarabine.


