MonotropeinCAS# 5945-50-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5945-50-6 | SDF | Download SDF |
| PubChem ID | 73466 | Appearance | White powder |
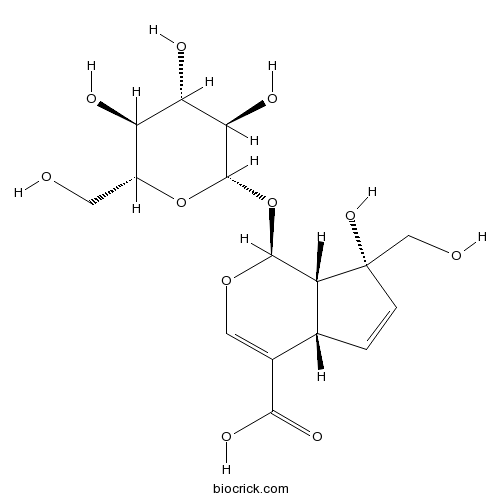
| Formula | C16H22O11 | M.Wt | 390.34 |
| Type of Compound | Iridoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in methanol and water | ||
| Chemical Name | (1S,4aS,7R,7aS)-7-hydroxy-7-(hydroxymethyl)-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4a,7a-dihydro-1H-cyclopenta[c]pyran-4-carboxylic acid | ||
| SMILES | C1=CC(C2C1C(=COC2OC3C(C(C(C(O3)CO)O)O)O)C(=O)O)(CO)O | ||
| Standard InChIKey | HPWWQPXTUDMRBI-NJPMDSMTSA-N | ||
| Standard InChI | InChI=1S/C16H22O11/c17-3-8-10(19)11(20)12(21)15(26-8)27-14-9-6(1-2-16(9,24)5-18)7(4-25-14)13(22)23/h1-2,4,6,8-12,14-15,17-21,24H,3,5H2,(H,22,23)/t6-,8-,9-,10-,11+,12-,14+,15+,16+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Monotropein has anti-antiosteoporosis and anti-inflammatory activities, it exerts protective effects against IL-1β-induced apoptosis and catabolic responses on osteoarthritis chondrocytes and can increase osteoblastic bone formation and prevent bone loss in ovariectomized mice. |
| Targets | MMP(e.g.TIMP) | IL Receptor | NOS | COX | TNF-α | NF-kB |
| In vitro | Monotropein exerts protective effects against IL-1β-induced apoptosis and catabolic responses on osteoarthritis chondrocytes.[Pubmed: 25466264]Int Immunopharmacol. 2014 Dec;23(2):575-80.Osteoarthritis, characterized by a loss of articular cartilage accompanied with inflammation, is the most common age-associated degenerative disease. Monotropein, an iridoids glycoside isolated from the roots of Morinda officinalis How, has been demonstrated to exhibit anti-inflammatory activity.
|
| In vivo | Monotropein isolated from the roots of Morinda officinalis ameliorates proinflammatory mediators in RAW 264.7 macrophages and dextran sulfate sodium (DSS)-induced colitis via NF-κB inactivation.[Pubmed: 23261679]Food Chem Toxicol. 2013 Mar;53:263-71.We previously demonstrated that Monotropein isolated from the roots of Morinda officinalis (Rubiaceae) has anti-inflammatory effects in vivo.
Monotropein isolated from the roots of Morinda officinalis increases osteoblastic bone formation and prevents bone loss in ovariectomized mice.[Pubmed: 26996879]Fitoterapia. 2016 Apr;110:166-72.Monotropein is a natural iridoid glycoside enriched in Morinda officinalis and has been used for medicinal purposes in China.
|
| Animal Research | Antinociceptive anti-inflammatory effect of Monotropein isolated from the root of Morinda officinalis.[Pubmed: 16204945]Biol Pharm Bull. 2005 Oct;28(10):1915-8.The root of Morinda officinalis (Rubiaceae) is used to treat rheumatoid arthritis and impotence in the traditional Oriental medicine. To identify the antinociceptive anti-inflammatory components of this crude drug, we adopted an activity-directed fractionation approach.
|

Monotropein Dilution Calculator

Monotropein Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5619 mL | 12.8093 mL | 25.6187 mL | 51.2374 mL | 64.0467 mL |
| 5 mM | 0.5124 mL | 2.5619 mL | 5.1237 mL | 10.2475 mL | 12.8093 mL |
| 10 mM | 0.2562 mL | 1.2809 mL | 2.5619 mL | 5.1237 mL | 6.4047 mL |
| 50 mM | 0.0512 mL | 0.2562 mL | 0.5124 mL | 1.0247 mL | 1.2809 mL |
| 100 mM | 0.0256 mL | 0.1281 mL | 0.2562 mL | 0.5124 mL | 0.6405 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1-Methyl-2-undecylquinolin-4(1H)-one
Catalog No.:BCN6591
CAS No.:59443-02-6
- 1F-Fructofuranosylnystose
Catalog No.:BCN8287
CAS No.:59432-60-9
- Boc-HoSer(Bzl)-OH
Catalog No.:BCC3244
CAS No.:59408-74-1
- Darutigenol
Catalog No.:BCN4096
CAS No.:5940-00-1
- PIK-93
Catalog No.:BCC2519
CAS No.:593960-11-3
- Alizapride HCl
Catalog No.:BCC4618
CAS No.:59338-87-3
- Triacontanol
Catalog No.:BCC8260
CAS No.:593-50-0
- Lindleyin
Catalog No.:BCN8450
CAS No.:59282-56-3
- Vitexin 2''-O-p-coumarate
Catalog No.:BCN2793
CAS No.:59282-55-2
- Sissotrin
Catalog No.:BCN4095
CAS No.:5928-26-7
- Decursin
Catalog No.:BCN5335
CAS No.:5928-25-6
- Acyclovir
Catalog No.:BCC3929
CAS No.:59277-89-3
- Nimbin
Catalog No.:BCN4617
CAS No.:5945-86-8
- Dihydropyrocurzerenone
Catalog No.:BCN8061
CAS No.:59462-26-9
- NSC 146109 hydrochloride
Catalog No.:BCC2410
CAS No.:59474-01-0
- Tafamidis
Catalog No.:BCC5268
CAS No.:594839-88-0
- Z-D-Glu(OBzl)-OH
Catalog No.:BCC2772
CAS No.:59486-73-6
- Citric acid monohydrate
Catalog No.:BCN8492
CAS No.:5949-29-1
- Testosterone undecanoate
Catalog No.:BCC9173
CAS No.:5949-44-0
- Calycanthine
Catalog No.:BCN7823
CAS No.:595-05-1
- Soyasapogenol B
Catalog No.:BCN4097
CAS No.:595-15-3
- Megestrol Acetate
Catalog No.:BCC4365
CAS No.:595-33-5
- Boc-Ser-OBzl
Catalog No.:BCC3440
CAS No.:59524-02-6
- H-D-Ala-OtBu.HCl
Catalog No.:BCC2849
CAS No.:59531-86-1
Monotropein exerts protective effects against IL-1beta-induced apoptosis and catabolic responses on osteoarthritis chondrocytes.[Pubmed:25466264]
Int Immunopharmacol. 2014 Dec;23(2):575-80.
Osteoarthritis, characterized by a loss of articular cartilage accompanied with inflammation, is the most common age-associated degenerative disease. Monotropein, an iridoids glycoside isolated from the roots of Morinda officinalis How, has been demonstrated to exhibit anti-inflammatory activity. In the present study, Monotropein was firstly to exhibit cartilage protective activity by down regulating the pro-inflammatory cytokines in the knee synovial fluid in vivo. The anti-apoptotic and anti-catabolic effects of Monotropein on rat OA chondrocytes treated by IL-1beta were investigated in vitro. In cultured chondrocytes, Monotropein attenuated apoptosis in a dose-dependent manner in response to IL-1beta stimulation. Moreover, treatment with Monotropein, the expressions of MMP-3 and MMP-13 were significantly decreased, the expression of COL2A1 was increased. Taken together, these findings suggested that Monotropein exerted anti-apoptosis and anti-catabolic activity in chondrocytes, which might support its possible therapeutic role in OA.
Antinociceptive anti-inflammatory effect of Monotropein isolated from the root of Morinda officinalis.[Pubmed:16204945]
Biol Pharm Bull. 2005 Oct;28(10):1915-8.
The root of Morinda officinalis (Rubiaceae) is used to treat rheumatoid arthritis and impotence in the traditional Oriental medicine. To identify the antinociceptive anti-inflammatory components of this crude drug, we adopted an activity-directed fractionation approach. The active fraction of the BuOH extract of M. officinalis root was subjected to silica gel and ODS column chromatography to yield two diterpenes, compounds 1 and 2 and these were identified as Monotropein and deacetylasperulosidic acid, respectively. The iridoid glycoside, Monotropein, was tested for its anti-inflammatory antinociceptive effects using hot plate- and writhing antinociceptive assays and by using carrageenan-induced anti-inflammatory assays in mice and rats. Pretreatment with Monotropein (at 20, 30 mg/kg/d, p.o.) significantly reduced stretching episodes and prolonged action time in mice. It also significantly reduced acute paw edema by carrageenan in rats. These results indicate that Monotropein contributes to the antinociceptive and anti-inflammatory action of Morinda officinalis root.
Monotropein isolated from the roots of Morinda officinalis ameliorates proinflammatory mediators in RAW 264.7 macrophages and dextran sulfate sodium (DSS)-induced colitis via NF-kappaB inactivation.[Pubmed:23261679]
Food Chem Toxicol. 2013 Mar;53:263-71.
We previously demonstrated that Monotropein isolated from the roots of Morinda officinalis (Rubiaceae) has anti-inflammatory effects in vivo. In the present study, we investigated the molecular mechanisms underlying the anti-inflammatory effects of Monotropein in lipopolysaccharide (LPS)-induced RAW 264.7 macrophages and dextran sulfate sodium (DSS)-induced colitis mouse model. Monotropein was found to inhibit the expressions of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), tumor necrosis factor-alpha (TNF-alpha), and interleukin-1beta (IL-1beta) mRNA in LPS-induced RAW 264.7 macrophages. Treatment with Monotropein decreased the DNA binding activity of nuclear factor-kappaB (NF-kappaB). Consistent with these findings, Monotropein also suppressed phosphorylation and degradation of inhibitory kappaB-alpha (IkappaB-alpha), and consequently the translocations of NF-kappaB. In the DSS-induced colitis model, Monotropein reduced disease activity index (DAI), myeloperoxidase (MPO) activity, and inflammation-related protein expressions by suppressing NF-kappaB activation in colon mucosa. Taken together, these findings suggest that the anti-inflammatory effects of Monotropein are mainly related to the inhibition of the expressions of inflammatory mediators via NF-kappaB inactivation, and support its possible therapeutic role in colitis.
Monotropein isolated from the roots of Morinda officinalis increases osteoblastic bone formation and prevents bone loss in ovariectomized mice.[Pubmed:26996879]
Fitoterapia. 2016 Apr;110:166-72.
Monotropein is a natural iridoid glycoside enriched in Morinda officinalis and has been used for medicinal purposes in China. In the present study, we systematically examined its effects on ovariectomy (OVX)-induced osteoporosis in mice and osteoblastic MC3T3-E1 cells for the first time. Eight-week-old female C57/BL6 mice were used to evaluate the osteoprotective effect of Monotropein. Results showed that administration of Monotropein (40 or 80 mg/kg/day) for four weeks exerted good bone protective effects as evidenced by the increase of bone mineral content (BMC), bone mineral density (BMD), bone volume fraction (BVF) and improvement of bone microstructure. Monotropein also enhanced the parameters of biomechanical properties, including maximum load, maximum stress and elastic modulus of femur in OVX mice. In addition, Monotropein treatment decreased the serum levels of interleukin 1 (IL-1), interleukin 6 (IL-6) and soluble receptor activator of NF-kappaB ligand (sRANKL) in OVX mice. In this study, we also assessed the effects of Monotropein on the proliferation and differentiation of osteoblastic MC3T3-E1 cells in vitro. After incubation for 48h, the cell proliferation was increased at the concentration of 10 muM, 25 muM, 50 muM and 100 muM. ALP activities were significantly increased after treatment with Monotropein for 72h. Quantitative analyses with alizarin red staining showed significantly increased mineralization of MC3T3-E1 cells after treatment with Monotropein for 28 days. Based on these results, Monotropein may serve as a new candidate or a leading compound for antiosteoporosis.


