DarutigenolCAS# 5940-00-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5940-00-1 | SDF | Download SDF |
| PubChem ID | 329240 | Appearance | Powder |
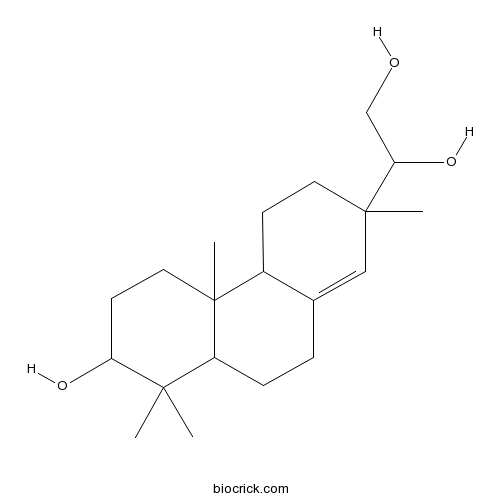
| Formula | C20H34O3 | M.Wt | 322.5 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-(7-hydroxy-2,4b,8,8-tetramethyl-4,4a,5,6,7,8a,9,10-octahydro-3H-phenanthren-2-yl)ethane-1,2-diol | ||
| SMILES | CC1(C2CCC3=CC(CCC3C2(CCC1O)C)(C)C(CO)O)C | ||
| Standard InChIKey | NCAZLDCEJHFJDT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H34O3/c1-18(2)15-6-5-13-11-19(3,17(23)12-21)9-7-14(13)20(15,4)10-8-16(18)22/h11,14-17,21-23H,5-10,12H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Darutigenol has obvious antithrombotic effect,its mechanism may be related to inhibition of platelet aggregation and adhesion. |

Darutigenol Dilution Calculator

Darutigenol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1008 mL | 15.5039 mL | 31.0078 mL | 62.0155 mL | 77.5194 mL |
| 5 mM | 0.6202 mL | 3.1008 mL | 6.2016 mL | 12.4031 mL | 15.5039 mL |
| 10 mM | 0.3101 mL | 1.5504 mL | 3.1008 mL | 6.2016 mL | 7.7519 mL |
| 50 mM | 0.062 mL | 0.3101 mL | 0.6202 mL | 1.2403 mL | 1.5504 mL |
| 100 mM | 0.031 mL | 0.155 mL | 0.3101 mL | 0.6202 mL | 0.7752 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- PIK-93
Catalog No.:BCC2519
CAS No.:593960-11-3
- Alizapride HCl
Catalog No.:BCC4618
CAS No.:59338-87-3
- Triacontanol
Catalog No.:BCC8260
CAS No.:593-50-0
- Lindleyin
Catalog No.:BCN8450
CAS No.:59282-56-3
- Vitexin 2''-O-p-coumarate
Catalog No.:BCN2793
CAS No.:59282-55-2
- Sissotrin
Catalog No.:BCN4095
CAS No.:5928-26-7
- Decursin
Catalog No.:BCN5335
CAS No.:5928-25-6
- Acyclovir
Catalog No.:BCC3929
CAS No.:59277-89-3
- Meptazinol HCl
Catalog No.:BCC4920
CAS No.:59263-76-2
- Rigosertib sodium
Catalog No.:BCC4067
CAS No.:592542-60-4
- Rigosertib
Catalog No.:BCC4296
CAS No.:592542-59-1
- Chikusetsu Saponin Ib
Catalog No.:BCC8308
CAS No.:59252-87-8
- Boc-HoSer(Bzl)-OH
Catalog No.:BCC3244
CAS No.:59408-74-1
- 1F-Fructofuranosylnystose
Catalog No.:BCN8287
CAS No.:59432-60-9
- 1-Methyl-2-undecylquinolin-4(1H)-one
Catalog No.:BCN6591
CAS No.:59443-02-6
- Monotropein
Catalog No.:BCN6280
CAS No.:5945-50-6
- Nimbin
Catalog No.:BCN4617
CAS No.:5945-86-8
- Dihydropyrocurzerenone
Catalog No.:BCN8061
CAS No.:59462-26-9
- NSC 146109 hydrochloride
Catalog No.:BCC2410
CAS No.:59474-01-0
- Tafamidis
Catalog No.:BCC5268
CAS No.:594839-88-0
- Z-D-Glu(OBzl)-OH
Catalog No.:BCC2772
CAS No.:59486-73-6
- Citric acid monohydrate
Catalog No.:BCN8492
CAS No.:5949-29-1
- Testosterone undecanoate
Catalog No.:BCC9173
CAS No.:5949-44-0
- Calycanthine
Catalog No.:BCN7823
CAS No.:595-05-1
[Study on index components and fingerprints of crude and processed Siegesbeckia Herbs].[Pubmed:25423830]
Zhongguo Zhong Yao Za Zhi. 2014 Aug;39(15):2907-11.
The change of kirenol, Darutigenol and darutoside in Siegesbeckia and its first to ninth processed products were studied, and the ten fingerprints were compared, which provided the experimental basis for the study of Siegesbeckia processing tech- nology. The samples were analysed by HPLC on a SunFire-C18 column (4.6 mm x 150 mm, 5 mum) with gradient elution of acetonitrile (0.1% formic acid)-water (0.1% formic acid) at a flow rate of 1.0 mL x min(-1). Column temperaturewas 30 degrees C and the detected wavelength was 215, 320 nm. The calibration curves of kirenol, Darutigenol and darutoside were linear in the range of 2.180-26.16, 2.900-34.80, and 1.012-6.072 mg x L(-1), respectively, and the average recoveries were 96.4%, 97.2% and 96.3% wit RSD 2.2%, 1.7% and 2.4%. This method was simple, the result was stable and had good repeatability, recovery and precision. The re- sult was the basis of the chemical contents variation in the processing of Siegesbeckia Herbs and further clarifying the effect of the changing.
Molecularly imprinted polymer for the specific solid-phase extraction of kirenol from Siegesbeckia pubescens herbal extract.[Pubmed:22284524]
Talanta. 2012 Jan 30;89:505-12.
Molecular imprinted polymers (MIPs) were prepared by thermal polymerization using a non-covalent molecularly imprinting strategy with kirenol as the template, acrylamide (AM) as the functional monomer and ethylene glycol dimethacrylamide (EGDMA) as the cross-linker in the porogen of tetrahydrofuran (THF). The synthesized MIPs were characterized by scanning electron microscopy (SEM) and Fourier transform infrared (FT-IR). Its molecular recognition property was investigated by UV spectrogram. High-pressure liquid chromatography (HPLC) was used for analysis of target analytes. The polymers were evaluated further by batch rebinding experiments, and from the derived isotherms their binding capacity and binding strength were determined. Then the selectivity of the MIPs was checked toward the selected structurally related compounds and the recognition coefficients for kirenol, Darutigenol, and ent-2-oxo-15, 16, 19-trihydroxypimar-8(14)-ene (TD) were 2.47, 3.43 and 3.40, respectively. The properties of MIPs for SPE were also evaluated. The results obtained demonstrate that the good imprinting effect and the excellent selectivity of MIPs were obtained. The optimized molecular imprinted SPE procedure was applied to extract kirenol directly from the extracts of the aerial part of Siegesbeckia pubescens herb. A selective extraction of kirenol from traditional Chinese medicine (TCM) was achieved with extraction yield of 80.9%.


