PMPA (NMDA antagonist)CAS# 113919-36-1 |
- Carboxypeptidase G2 (CPG2) Inhibitor
Catalog No.:BCC1452
CAS No.:192203-60-4
- CPA inhibitor
Catalog No.:BCC1500
CAS No.:223532-02-3
- 2-MPPA
Catalog No.:BCC7995
CAS No.:254737-29-6
- ZJ 43
Catalog No.:BCC2355
CAS No.:723331-20-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 113919-36-1 | SDF | Download SDF |
| PubChem ID | 17756792 | Appearance | Powder |
| Formula | C6H13N2O5P | M.Wt | 224.15 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | PMPC | ||
| Solubility | Soluble to 100 mM in water | ||
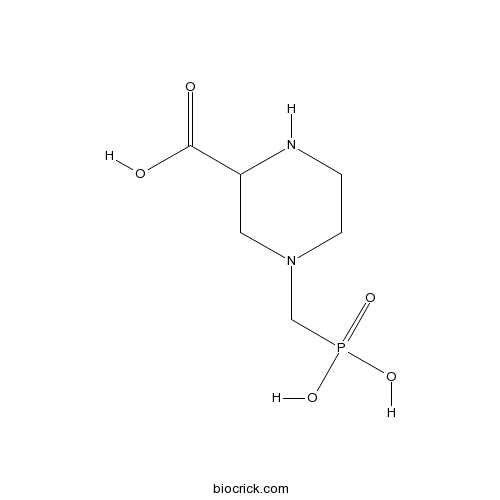
| Chemical Name | 4-(phosphonomethyl)piperazine-2-carboxylic acid | ||
| SMILES | C1CN(CC(N1)C(=O)O)CP(=O)(O)O | ||
| Standard InChIKey | ILRBBHJXTYIHTP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H13N2O5P/c9-6(10)5-3-8(2-1-7-5)4-14(11,12)13/h5,7H,1-4H2,(H,9,10)(H2,11,12,13) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Competitive NMDA receptor antagonist. Displays Ki values of 0.84, 2.74, 3.53 and 4.16 μM at NR2A, NR2B, NR2C and NR2D subunit-containing receptors respectively. Selective over AMPA receptors. |

PMPA (NMDA antagonist) Dilution Calculator

PMPA (NMDA antagonist) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4613 mL | 22.3065 mL | 44.613 mL | 89.226 mL | 111.5325 mL |
| 5 mM | 0.8923 mL | 4.4613 mL | 8.9226 mL | 17.8452 mL | 22.3065 mL |
| 10 mM | 0.4461 mL | 2.2306 mL | 4.4613 mL | 8.9226 mL | 11.1532 mL |
| 50 mM | 0.0892 mL | 0.4461 mL | 0.8923 mL | 1.7845 mL | 2.2306 mL |
| 100 mM | 0.0446 mL | 0.2231 mL | 0.4461 mL | 0.8923 mL | 1.1153 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- PNU 74654
Catalog No.:BCC7704
CAS No.:113906-27-7
- Koumine N-oxide
Catalog No.:BCN4807
CAS No.:113900-75-7
- Caryophyllene oxide
Catalog No.:BCN6019
CAS No.:1139-30-6
- Cinnamyl 3-aminobut-2-enoate
Catalog No.:BCC8914
CAS No.:113898-97-8
- CX-5461
Catalog No.:BCC3700
CAS No.:1138549-36-6
- Cidofovir
Catalog No.:BCC2546
CAS No.:113852-37-2
- N-p-coumaroyl-N'-caffeoylputrescine
Catalog No.:BCN6018
CAS No.:1138156-77-0
- N1,N12-Diethylspermine tetrahydrochloride
Catalog No.:BCC6669
CAS No.:113812-15-0
- Picrasidine T
Catalog No.:BCN6017
CAS No.:113808-03-0
- Z-Gly-OH
Catalog No.:BCC2770
CAS No.:1138-80-3
- (Z)-FeCP-oxindole
Catalog No.:BCC6079
CAS No.:1137967-28-2
- TAK960
Catalog No.:BCC6411
CAS No.:1137868-52-0
- Andrographidine C
Catalog No.:BCN4730
CAS No.:113963-39-6
- Andrographidine E
Catalog No.:BCN4729
CAS No.:113963-41-0
- (Z)-Akuammidine
Catalog No.:BCN6020
CAS No.:113973-31-2
- Cefozopran hydrochloride
Catalog No.:BCC8909
CAS No.:113981-44-5
- 5-Hydroxy-7,8-dimethoxyflavanone
Catalog No.:BCN6021
CAS No.:113981-49-0
- Golgicide A
Catalog No.:BCC4373
CAS No.:1139889-93-2
- Erythromycin
Catalog No.:BCC4778
CAS No.:114-07-8
- Scopolamine hydrobromide
Catalog No.:BCN1199
CAS No.:114-49-8
- Neostigmine Bromide
Catalog No.:BCC4563
CAS No.:114-80-7
- Phenformin
Catalog No.:BCC9120
CAS No.:114-86-3
- Phaclofen
Catalog No.:BCC6562
CAS No.:114012-12-3
- 16-Epivoacarpine
Catalog No.:BCN3940
CAS No.:114027-38-2
The effect of competitive antagonist chain length on NMDA receptor subunit selectivity.[Pubmed:15721167]
Neuropharmacology. 2005 Mar;48(3):354-9.
The widely-used N-methyl-D-aspartate (NMDA) receptor antagonists (R)-4-(3-phosphonopropyl) piperazine-2-carboxylic acid ((R)-CPP) and (R)-2-amino-7-phosphonoheptanoate ((R)-AP7) are frequently used as general NMDA receptor antagonists and assumed not to display significant selectivity among NMDA receptor NR2 subunits. However, electrophysiological studies have suggested that certain longer chain N-methyl-D-aspartate (NMDA) receptor competitive antagonists, such as (R)-CPP are ineffective at subpopulations of NMDA receptors in the red nucleus, superior colliculus, and hippocampus. Using recombinant receptors expressed in Xenopus oocytes, we have examined the effect of antagonist chain length on NR2 subunit selectivity. All antagonists displayed the potency order (high to low affinity) of NR2A > NR2B > NR2C > NR2D, however the longer chain antagonists (having 7 instead of 5 bond lengths between acidic groups) displayed much greater subunit selectivity than their short-chain homologues. For example (R)-CPP displayed a 50-fold difference in affinity between NR2A-containing and NR2D-containing NMDA receptors, while the shorter chain homologue 4-(phosphonomethyl) piperazine-2-carboxylic acid (PMPA) displayed only a 5-fold variation in affinity. These results can account for the earlier physiological findings and suggest that longer chain antagonists such as (R)-CPP and (R)-AP7 should not be used as general NMDA receptor antagonists.
Cortically evoked excitatory synaptic transmission in the cat red nucleus is antagonised by D-AP5 but not by D-AP7.[Pubmed:1361408]
Brain Res. 1992 Oct 23;594(1):176-80.
Extracellular recordings were made from magnocellular red nucleus neurons (mRN) in alpha-chloralose (50 mg/kg, iv.) anaesthetised cats. Iontophoretically applied N-methyl-D-aspartate (NMDA) excited the neuronal firing which was antagonised by 4 selective NMDA receptor antagonists: 2-amino-5-phosphonopentanoate (AP5), 2-amino-7-phosphonoheptanoate (AP7), RS-4-(phosphonomethyl) piperazine-2-carboxylic acid (PMPC) and R-4-(3-phosphonopropyl) piperazine-2-carboxylic acid (CPP), whereas AMPA responses were uneffected. Monosynaptic excitatory responses were produced by stimulation of the sensorimotor cortex. These responses were reduced and often abolished by AP5 and PMPC but not by AP7 or CPP. It is postulated that two NMDA receptor subtypes exist on mRN neurones.


