2-MPPASelective glutamate carboxypeptidase II (GCP II) inhibitor; orally bioavailable CAS# 254737-29-6 |
- ABT-751 (E7010)
Catalog No.:BCC1085
CAS No.:141430-65-1
- CHM 1
Catalog No.:BCC2387
CAS No.:154554-41-3
- Podophyllotoxin
Catalog No.:BCN5957
CAS No.:518-28-5
- D-64131
Catalog No.:BCC1510
CAS No.:74588-78-6
- CYT997 (Lexibulin)
Catalog No.:BCC4601
CAS No.:917111-44-5
- MPC 6827 hydrochloride
Catalog No.:BCC8040
CAS No.:917369-31-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 254737-29-6 | SDF | Download SDF |
| PubChem ID | 10198171 | Appearance | Powder |
| Formula | C8H14O4S | M.Wt | 206.26 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
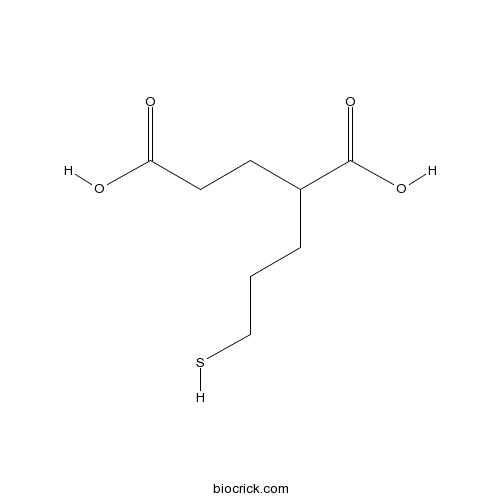
| Chemical Name | 2-(3-sulfanylpropyl)pentanedioic acid | ||
| SMILES | C(CC(CCC(=O)O)C(=O)O)CS | ||
| Standard InChIKey | FNLNSQHJKVQCBP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H14O4S/c9-7(10)4-3-6(8(11)12)2-1-5-13/h6,13H,1-5H2,(H,9,10)(H,11,12) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective glutamate carboxypeptidase II (GCP II) inhibitor (IC50 = 90 nM). Selective for GCP II over NMDA, kainate and AMPA glutamate receptors and MMP-1, -2, -3, -7, -9, ACE and NEP metalloproteases. Antinociceptive in a rat model of neuropathic pain and protects against motor neuron death in familial amyotrophic lateral sclerosis mice. Orally bioavailable. |

2-MPPA Dilution Calculator

2-MPPA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8482 mL | 24.2412 mL | 48.4825 mL | 96.965 mL | 121.2062 mL |
| 5 mM | 0.9696 mL | 4.8482 mL | 9.6965 mL | 19.393 mL | 24.2412 mL |
| 10 mM | 0.4848 mL | 2.4241 mL | 4.8482 mL | 9.6965 mL | 12.1206 mL |
| 50 mM | 0.097 mL | 0.4848 mL | 0.9696 mL | 1.9393 mL | 2.4241 mL |
| 100 mM | 0.0485 mL | 0.2424 mL | 0.4848 mL | 0.9696 mL | 1.2121 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Demethyleneberberine
Catalog No.:BCN2829
CAS No.:25459-91-0
- H-Asn-OtBu
Catalog No.:BCC2877
CAS No.:25456-86-4
- H-D-Glu-OtBu
Catalog No.:BCC2938
CAS No.:25456-76-2
- Felbamate
Catalog No.:BCC4904
CAS No.:25451-15-4
- (+)-Afzelechin
Catalog No.:BCN5121
CAS No.:2545-00-8
- Dehydropitavastatin ethyl ester
Catalog No.:BCC8931
CAS No.:254452-91-0
- H-Cys(pMeOBzl)-OH
Catalog No.:BCC2909
CAS No.:2544-31-2
- Phellopterin
Catalog No.:BCN2637
CAS No.:2543-94-4
- Boc-ß-HoAsp(OBzl)-OH
Catalog No.:BCC3229
CAS No.:254101-10-5
- Morroniside
Catalog No.:BCN5009
CAS No.:25406-64-8
- Kanamycin Sulfate
Catalog No.:BCC1205
CAS No.:25389-94-0
- AR-C 102222
Catalog No.:BCC6092
CAS No.:253771-21-0
- Emricasan
Catalog No.:BCC5367
CAS No.:254750-02-2
- Talsupram hydrochloride
Catalog No.:BCC7924
CAS No.:25487-28-9
- Curryangine
Catalog No.:BCN7907
CAS No.:25488-37-3
- 2,3-Bis(3,4-dimethoxybenzyl)butyrolactone
Catalog No.:BCN1473
CAS No.:25488-59-9
- Kushenol W
Catalog No.:BCN3307
CAS No.:254886-76-5
- Kushenol X
Catalog No.:BCN3350
CAS No.:254886-77-6
- 4-Allyloxy-2-hydroxybenzophenone
Catalog No.:BCC8675
CAS No.:2549-87-3
- Tasquinimod
Catalog No.:BCC1987
CAS No.:254964-60-8
- 3-Epioleanolic acid
Catalog No.:BCN3050
CAS No.:25499-90-5
- 4-Phenylbutan-2-one
Catalog No.:BCN3808
CAS No.:2550-26-7
- 1-Acetyl-4-piperidinecarboxylic acid
Catalog No.:BCC8447
CAS No.:25503-90-6
- Bruceine B
Catalog No.:BCN7615
CAS No.:25514-29-8
Pharmacokinetics and pharmacodynamics of the glutamate carboxypeptidase II inhibitor 2-MPPA show prolonged alleviation of neuropathic pain through an indirect mechanism.[Pubmed:23776202]
J Pharmacol Exp Ther. 2013 Sep;346(3):406-13.
Glutamate carboxypeptidase II (GCP II) is a therapeutic target in neurologic disorders associated with excessive activation of glutamatergic systems. The potent, orally bioavailable GCP II inhibitor 2-(3-mercaptopropyl) pentanedioic acid (2-MPPA) is effective in preclinical models of diseases where excess glutamate release is implicated, including neuropathic pain, and was the first GCP II inhibitor to be administered to man. The relationships between dosing regimen, pharmacokinetics, and analgesia in a neuropathic pain model were examined in rats to aid development of clinical dosing. The efficacy of oral 2-MPPA in the chronic constrictive injury model was not simply related to plasma concentrations. Even though maximal concentrations were observed within 1 hour of dosing, the analgesic effect took at least 8 days of daily dosing to become significant. The delay was not due to tissue drug accumulation since inhibitory concentrations of the drug were achieved in the nerve within 1 hour of dosing. There was also no accumulation of drug in plasma or tissue after multiple daily dosing. Effects were dependent on reaching a threshold concentration since dividing the daily dose led to a loss of effect. The analgesic effect outlasted plasma exposure and was maintained for days even after daily dosing was halted. The delayed onset, dependence on threshold plasma concentration, and sustained effects after exposure support the hypothesis that an indirect, long-lived mechanism of action exists. Although these longer lasting secondary mechanisms are not yet identified, daily clinical dosing of a GCP II inhibitor seems justified.
2-MPPA, a selective glutamate carboxypeptidase II inhibitor, attenuates morphine tolerance but not dependence in C57/Bl mice.[Pubmed:16220328]
Psychopharmacology (Berl). 2005 Dec;183(3):275-84.
RATIONALE AND OBJECTIVES: We have recently reported that conditioned morphine reward and tolerance to its antinociceptive effect, but not expression of morphine dependence, were attenuated by 2-(phosphonomethyl)pentanedioic acid (2-PMPA), a prototypic inhibitor of glutamate carboxipeptidase II (GCP II), which is an enzyme responsible for the supply of glutamate. In the present study, we investigated in more detail the effects of GCP II inhibition on opioid dependence and tolerance to its antinociceptive effect in C57/Bl mice using a novel GCP II inhibitor. RESULTS: The treatment with 2-(3-mercaptopropyl)pentanedioic acid (2-MPPA; 60 but not 10 or 30 mg/kg) prevented the development of morphine tolerance without affecting acute morphine antinociception. 2-MPPA at 30 and 60 mg/kg did not prevent the development of dependence induced by 10 and 30 mg/kg of morphine. The study on opioid withdrawal syndrome, i.e., expression of opioid dependence, demonstrated that 2-MPPA potentiated jumping behavior and teeth chattering but attenuated chewing and ptosis. None of these opioid withdrawal signs were affected by 2-MPPA in morphine nondependent mice. Pretreatment with the mGluR II antagonist LY341495 (1 mg/kg) reversed the 2-MPPA-induced increase or decrease in opioid withdrawal signs in morphine-dependent mice. 2-MPPA (60 mg/kg) administered for 7 days with morphine did not affect brain concentration of this opiate. CONCLUSIONS: The present findings suggest complex effects of GCP II inhibition on morphine dependence and tolerance and imply a role of mGluR II in the actions of 2-MPPA.
Orally active glutamate carboxypeptidase II inhibitor 2-MPPA attenuates dizocilpine-induced prepulse inhibition deficits in mice.[Pubmed:21093418]
Brain Res. 2011 Jan 31;1371:82-6.
Glutamate carboxypeptidase II (GCP II) is a glial enzyme responsible for the hydrolysis of N-acetylaspartylglutamate (NAAG) into glutamate and N-acetylaspartate (NAA). Abnormalities in glutamate neurotransmission are implicated in the pathophysiology of schizophrenia. In this study, we examined the effects of a novel, orally active GCP II inhibitor, 2-(3-mercaptopropyl)pentanedioic acid (2-MPPA), on the prepulse inhibition (PPI) deficits after administration of the N-methyl-d-aspartate (NMDA) receptor antagonist dizocilpine. Oral administration of 2-MPPA (10, 30 or 100mg/kg) significantly attenuated dizocilpine (0.1mg/kg)-induced PPI deficits in mice, in a dose dependent manner. Furthermore, the efficacy of 2-MPPA on dizocilpine-induced PPI deficits was significantly antagonized by pretreatment with the selective group II metabotropic glutamate receptor (mGluR) antagonist LY341495 (1.0mg/kg). In the same model, however, the selective group II mGluR agonist LY354740 (3, 10 or 30 mg/kg) significantly attenuated dizocilpine-induced PPI deficits at only one dose and prepulse intensity. Our findings suggest that GCP II inhibition may be useful therapeutic strategy for schizophrenia. From a mechanistic perspective, while increased NAAG and activation of group II mGluRs may contribute to the therapeutic efficacy of 2-MPPA, it is likely that additional pharmacological activities are also involved.
Glutamate carboxypeptidase II inhibition protects motor neurons from death in familial amyotrophic lateral sclerosis models.[Pubmed:12876198]
Proc Natl Acad Sci U S A. 2003 Aug 5;100(16):9554-9.
Approximately 10% of cases of amyotrophic lateral sclerosis (ALS), a progressive and fatal degeneration that targets motor neurons (MNs), are inherited, and approximately 20% of these cases of familial ALS (FALS) are caused by mutations of copper/zinc superoxide dismutase type 1. Glutamate excitotoxicity has been implicated as a mechanism of MN death in both ALS and FALS. In this study, we tested whether a neuroprotective strategy involving potent and selective inhibitors of glutamate carboxypeptidase II (GCPII), which converts the abundant neuropeptide N-acetylaspartylglutamate to glutamate, could protect MNs in an in vitro and animal model of FALS. Data suggest that the GCPII inhibitors prevented MN cell death in both of these systems because of the resultant decrease in glutamate levels. GCPII inhibition may represent a new therapeutic target for the treatment of ALS.
Synthesis and biological evaluation of thiol-based inhibitors of glutamate carboxypeptidase II: discovery of an orally active GCP II inhibitor.[Pubmed:12723961]
J Med Chem. 2003 May 8;46(10):1989-96.
A series of 2-(thioalkyl)pentanedioic acids were synthesized and evaluated as inhibitors of glutamate carboxypeptidase II (GCP II, EC 3.4.17.21). The inhibitory potency of these thiol-based compounds against GCP II was found to be dependent on the number of methylene units between the thiol group and pentanedioic acid. A comparison of the SAR of the thiol-based inhibitors to that of the phosphonate-based inhibitors provides insight into the role of each of the two zinc-binding groups in GCP II inhibition. The most potent thiol-based inhibitor, 2-(3-mercaptopropyl)pentanedioic acid (IC(50) = 90 nM), was found to be orally bioavailable in rats and exhibited efficacy in an animal model of neuropathic pain following oral administration.


