EmricasanCAS# 254750-02-2 |
- GPR40 Activator 1
Catalog No.:BCC4125
CAS No.:1309435-60-6
- TUG-770
Catalog No.:BCC2018
CAS No.:1402601-82-4
- Imeglimin hydrochloride
Catalog No.:BCC4085
CAS No.:775351-61-6
- Imeglimin
Catalog No.:BCC4221
CAS No.:775351-65-0
- Teneligliptin hydrobromide
Catalog No.:BCC1992
CAS No.:906093-29-6
- Chlorpropamide
Catalog No.:BCC4647
CAS No.:94-20-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

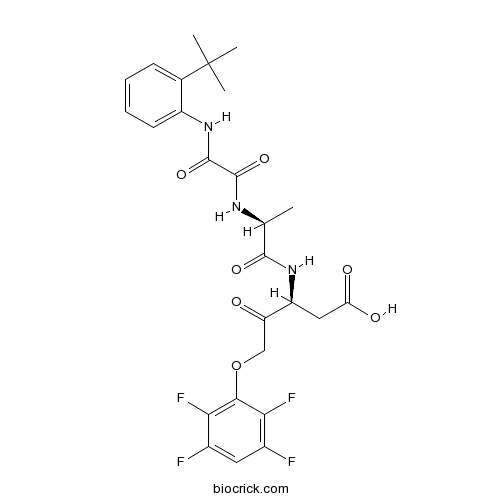
| Cas No. | 254750-02-2 | SDF | Download SDF |
| PubChem ID | 12000240 | Appearance | Powder |
| Formula | C26H27F4N3O7 | M.Wt | 569.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 42 mg/mL (73.75 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (3S)-3-[[(2S)-2-[[2-(2-tert-butylanilino)-2-oxoacetyl]amino]propanoyl]amino]-4-oxo-5-(2,3,5,6-tetrafluorophenoxy)pentanoic acid | ||
| SMILES | CC(C(=O)NC(CC(=O)O)C(=O)COC1=C(C(=CC(=C1F)F)F)F)NC(=O)C(=O)NC2=CC=CC=C2C(C)(C)C | ||
| Standard InChIKey | SCVHJVCATBPIHN-SJCJKPOMSA-N | ||
| Standard InChI | InChI=1S/C26H27F4N3O7/c1-12(31-24(38)25(39)32-16-8-6-5-7-13(16)26(2,3)4)23(37)33-17(10-19(35)36)18(34)11-40-22-20(29)14(27)9-15(28)21(22)30/h5-9,12,17H,10-11H2,1-4H3,(H,31,38)(H,32,39)(H,33,37)(H,35,36)/t12-,17-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Emricasan Dilution Calculator

Emricasan Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7559 mL | 8.7796 mL | 17.5593 mL | 35.1185 mL | 43.8982 mL |
| 5 mM | 0.3512 mL | 1.7559 mL | 3.5119 mL | 7.0237 mL | 8.7796 mL |
| 10 mM | 0.1756 mL | 0.878 mL | 1.7559 mL | 3.5119 mL | 4.3898 mL |
| 50 mM | 0.0351 mL | 0.1756 mL | 0.3512 mL | 0.7024 mL | 0.878 mL |
| 100 mM | 0.0176 mL | 0.0878 mL | 0.1756 mL | 0.3512 mL | 0.439 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-MPPA
Catalog No.:BCC7995
CAS No.:254737-29-6
- Demethyleneberberine
Catalog No.:BCN2829
CAS No.:25459-91-0
- H-Asn-OtBu
Catalog No.:BCC2877
CAS No.:25456-86-4
- H-D-Glu-OtBu
Catalog No.:BCC2938
CAS No.:25456-76-2
- Felbamate
Catalog No.:BCC4904
CAS No.:25451-15-4
- (+)-Afzelechin
Catalog No.:BCN5121
CAS No.:2545-00-8
- Dehydropitavastatin ethyl ester
Catalog No.:BCC8931
CAS No.:254452-91-0
- H-Cys(pMeOBzl)-OH
Catalog No.:BCC2909
CAS No.:2544-31-2
- Phellopterin
Catalog No.:BCN2637
CAS No.:2543-94-4
- Boc-ß-HoAsp(OBzl)-OH
Catalog No.:BCC3229
CAS No.:254101-10-5
- Morroniside
Catalog No.:BCN5009
CAS No.:25406-64-8
- Kanamycin Sulfate
Catalog No.:BCC1205
CAS No.:25389-94-0
- Talsupram hydrochloride
Catalog No.:BCC7924
CAS No.:25487-28-9
- Curryangine
Catalog No.:BCN7907
CAS No.:25488-37-3
- 2,3-Bis(3,4-dimethoxybenzyl)butyrolactone
Catalog No.:BCN1473
CAS No.:25488-59-9
- Kushenol W
Catalog No.:BCN3307
CAS No.:254886-76-5
- Kushenol X
Catalog No.:BCN3350
CAS No.:254886-77-6
- 4-Allyloxy-2-hydroxybenzophenone
Catalog No.:BCC8675
CAS No.:2549-87-3
- Tasquinimod
Catalog No.:BCC1987
CAS No.:254964-60-8
- 3-Epioleanolic acid
Catalog No.:BCN3050
CAS No.:25499-90-5
- 4-Phenylbutan-2-one
Catalog No.:BCN3808
CAS No.:2550-26-7
- 1-Acetyl-4-piperidinecarboxylic acid
Catalog No.:BCC8447
CAS No.:25503-90-6
- Bruceine B
Catalog No.:BCN7615
CAS No.:25514-29-8
- Bruceine C
Catalog No.:BCN8000
CAS No.:25514-30-1
Carcinogenicity assessment of the pan-caspase inhibitor, emricasan, in Tg.rasH2 mice.[Pubmed:25896096]
Regul Toxicol Pharmacol. 2015 Jul;72(2):169-78.
Emricasan, formerly IDN-6556, is a small molecule currently being evaluated in clinical trials to reduce hepatic injury and liver fibrosis. Since Emricasan is an irreversible pan-caspase inhibitor that potently inhibits caspase-mediated apoptosis and inflammation, its carcinogenic potential was evaluated in a humanized mouse model. Tg.rasH2 mice received LabDiet formulated with 0, 10, 25, and 75mg/kg/day of Emricasan, for 26weeks. At terminal sacrifice, blood was collected for clinical pathology analysis and tissues were collected, processed, and evaluated microscopically. There were no treatment related deaths or overt signs of toxicity for the duration of the study. There was no evidence of a carcinogenic effect in the peripheral blood leukocyte counts. Liver microgranulomas, which are background lesions, were slightly increased, especially in males. Increases in the incidence of the activated germinal centers were seen in the spleens and mesenteric lymph nodes of males and females, and in the mandibular lymph nodes of male mice. Atrophy of ovaries and testicular degeneration were also seen in Emricasan treated animals. Although several non-neoplastic lesions were observed, there was no evidence of Emricasan-related tumor formation in any tissue. In addition, the non-neoplastic lesions were not considered pre-neoplastic. Thus, Emricasan is not considered carcinogenic.
The pan-caspase inhibitor Emricasan (IDN-6556) decreases liver injury and fibrosis in a murine model of non-alcoholic steatohepatitis.[Pubmed:24750664]
Liver Int. 2015 Mar;35(3):953-66.
BACKGROUND & AIMS: Hepatocyte apoptosis, the hallmark of non-alcoholic steatohepatitis (NASH) contributes to liver injury and fibrosis. Although, both the intrinsic and extrinsic apoptotic pathways are involved in the pathogenesis of NASH, the final common step of apoptosis is executed by a family of cysteine-proteases termed caspases. Thus, our aim was to ascertain if administration of Emricasan, a pan-caspase inhibitor, ameliorates liver injury and fibrosis in a murine model of NASH. METHODS: C57/BL6J-mice were fed regular chow or high fat diet (HFD) for 20 weeks. All mice were treated with vehicle or Emricasan. RESULTS: Mice fed a HFD diet demonstrate a five-fold increase in hepatocyte apoptosis by the TUNEL assay and a 1.5-fold and 1.3-fold increase in caspase-3 and-8 activities respectively; this increase in apoptosis was substantially attenuated in mice fed a HFD treated with Emricasan (HFD-Em). Likewise, liver injury and inflammation were reduced in mice fed HFD-Em as compare to HFD by measuring serum aspartate aminotransferase and alanine aminotransferase levels, NAS histological score and IL 1-beta, TNF-alpha, monocyte chemoattractant protein (MCP-1) and C-X-C chemokine ligand-2 (CXCL2) quantitative reverse-transcription polymerase chain reaction (qPCR). These differences could not be attributed to differences in hepatic steatosis as liver triglycerides content were similar in both HFD groups. Hepatic fibrosis was reduced by Emricasan in HFD animals by decreasing alphaSMA (a marker for hepatic stellate cell activation), fibrosis score, Sirius red staining, hydroxyproline liver content and profibrogenic cytokines by qPCR. CONCLUSION: In conclusion, these data demonstrate that in a murine model of NASH, liver injury and fibrosis are suppressed by inhibiting hepatocytes apoptosis and suggests that Emricasan may be an attractive antifibrotic therapy in NASH.
The caspase-8 inhibitor emricasan combines with the SMAC mimetic birinapant to induce necroptosis and treat acute myeloid leukemia.[Pubmed:27194727]
Sci Transl Med. 2016 May 18;8(339):339ra69.
Resistance to chemotherapy is a major problem in cancer treatment, and it is frequently associated with failure of tumor cells to undergo apoptosis. Birinapant, a clinical SMAC mimetic, had been designed to mimic the interaction between inhibitor of apoptosis proteins (IAPs) and SMAC/Diablo, thereby relieving IAP-mediated caspase inhibition and promoting apoptosis of cancer cells. We show that acute myeloid leukemia (AML) cells are sensitive to birinapant-induced death and that the clinical caspase inhibitor Emricasan/IDN-6556 augments, rather than prevents, killing by birinapant. Deletion of caspase-8 sensitized AML to birinapant, whereas combined loss of caspase-8 and the necroptosis effector MLKL (mixed lineage kinase domain-like) prevented birinapant/IDN-6556-induced death, showing that inhibition of caspase-8 sensitizes AML cells to birinapant-induced necroptosis. However, loss of MLKL alone did not prevent a caspase-dependent birinapant/IDN-6556-induced death, implying that AML will be less likely to acquire resistance to this drug combination. A therapeutic breakthrough in AML has eluded researchers for decades. Demonstrated antileukemic efficacy and safety of the birinapant/Emricasan combination in vivo suggest that induction of necroptosis warrants clinical investigation as a therapeutic opportunity in AML.


