GPR40 Activator 1CAS# 1309435-60-6 |
- GPR120 modulator 1
Catalog No.:BCC1599
CAS No.:1050506-75-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1309435-60-6 | SDF | Download SDF |
| PubChem ID | 52936292 | Appearance | Powder |
| Formula | C31H31NO3S | M.Wt | 497.65 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
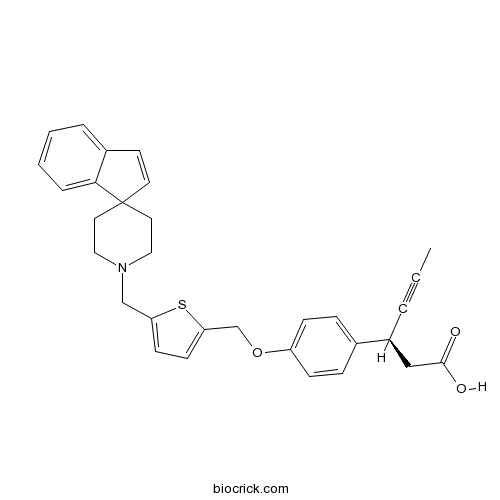
| Chemical Name | (3S)-3-[4-[[5-(spiro[indene-1,4'-piperidine]-1'-ylmethyl)thiophen-2-yl]methoxy]phenyl]hex-4-ynoic acid | ||
| SMILES | CC#CC(CC(=O)O)C1=CC=C(C=C1)OCC2=CC=C(S2)CN3CCC4(CC3)C=CC5=CC=CC=C45 | ||
| Standard InChIKey | YSVQUSMRABAJAR-VWLOTQADSA-N | ||
| Standard InChI | InChI=1S/C31H31NO3S/c1-2-5-25(20-30(33)34)23-8-10-26(11-9-23)35-22-28-13-12-27(36-28)21-32-18-16-31(17-19-32)15-14-24-6-3-4-7-29(24)31/h3-4,6-15,25H,16-22H2,1H3,(H,33,34)/t25-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GPR40 Activator 1 is a potent GPR40 activator for treatment of type 2 diabetes.
IC50 value:
Target: GPR40
Preparation of spiropiperidine derivatives for use as antidiabetic agents
By Hamdouchi, Chafiq; Lineswala, Jayana Pankaj; Maiti, Pranab
From PCT Int. Appl. (2011), WO 2011066183 A1 20110603. References: | |||||

GPR40 Activator 1 Dilution Calculator

GPR40 Activator 1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0094 mL | 10.0472 mL | 20.0944 mL | 40.1889 mL | 50.2361 mL |
| 5 mM | 0.4019 mL | 2.0094 mL | 4.0189 mL | 8.0378 mL | 10.0472 mL |
| 10 mM | 0.2009 mL | 1.0047 mL | 2.0094 mL | 4.0189 mL | 5.0236 mL |
| 50 mM | 0.0402 mL | 0.2009 mL | 0.4019 mL | 0.8038 mL | 1.0047 mL |
| 100 mM | 0.0201 mL | 0.1005 mL | 0.2009 mL | 0.4019 mL | 0.5024 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GPR40 Activator 1 is a potent GPR40 activator for treatment of type 2 diabetes.
- Cerberic acid B
Catalog No.:BCN4715
CAS No.:1309362-77-3
- CX-4945 sodium salt
Catalog No.:BCC5586
CAS No.:1309357-15-0
- Entacapone
Catalog No.:BCC2217
CAS No.:130929-57-6
- 7-O-Acetylneocaesalpin N
Catalog No.:BCN7332
CAS No.:1309079-08-0
- N4-Benzoylcytidine
Catalog No.:BCC9072
CAS No.:13089-48-0
- Fmoc-Val-OSu
Catalog No.:BCC3572
CAS No.:130878-68-1
- 1beta,10beta-Epoxydehydroleucodin
Catalog No.:BCN7331
CAS No.:130858-00-3
- ent-kaurane-3,16,17-triol
Catalog No.:BCN6164
CAS No.:130855-22-0
- Decursinol angelate
Catalog No.:BCC9222
CAS No.:130848-06-5
- Scoparinol
Catalog No.:BCN6163
CAS No.:130838-00-5
- 7-Oxohinokinin
Catalog No.:BCN6162
CAS No.:130837-92-2
- Pseudolarifuroic acid
Catalog No.:BCN8048
CAS No.:130825-79-5
- K145
Catalog No.:BCC4305
CAS No.:1309444-75-4
- Wittifuran X
Catalog No.:BCN4794
CAS No.:1309478-07-6
- Marumoside A
Catalog No.:BCN7702
CAS No.:1309604-34-9
- H 89 2HCl
Catalog No.:BCC4997
CAS No.:130964-39-5
- (±)-CPSI 1306
Catalog No.:BCC6161
CAS No.:1309793-47-2
- Sephin1
Catalog No.:BCC3980
CAS No.:13098-73-2
- MRS 4062 triethylammonium salt
Catalog No.:BCC6134
CAS No.:1309871-50-8
- 15-Methoxymkapwanin
Catalog No.:BCN6498
CAS No.:1309920-99-7
- VU 0360172 hydrochloride
Catalog No.:BCC6141
CAS No.:1309976-62-2
- alpha-Yohimbine
Catalog No.:BCN6166
CAS No.:131-03-3
- Dimethyl phthalate
Catalog No.:BCN6167
CAS No.:131-11-3
- Pimpinellin
Catalog No.:BCN6168
CAS No.:131-12-4
GPR40 mediates potential positive effects of a saturated fatty acid enriched diet on bone.[Pubmed:27611773]
Mol Nutr Food Res. 2017 Feb;61(2).
SCOPE: The stimulation of the free fatty acid receptor G-protein coupled receptor (GPR) 40 by GW9508 prevents bone loss by inhibiting osteoclast activity, both in vitro and in vivo. Here, we questioned whether the stimulation of the GPR40 receptor by dietary fatty acids may lead to the same beneficial effect on bone. METHODS AND RESULTS: We investigated (i) the impact of a fatty acid enriched diet (high-fat diet [HFD]) on bone health in C57/BL6 female mice depending on (ii) the estrogen status (ovariectomy) and (iii) the genotype (GPR40(+/+) or GPR40(-/-) ). Bone mineral density (BMD), body composition, weight, inflammation and bone remodeling parameters were monitored. HFD decreased BMD in HFD-SH-GPR40(+/+) mice but OVX failed to further impact BMD in HFD-OVX-GPR40(+/+) mice, while additional bone loss was observed in HFD-OVX-GPR40(-/-) animals. These data suggest that when stimulated by fatty acid enriched diets GPR40 contributes to counteract ovariectomy-induced bone alteration. The sparing effect is supported by the modulation of both the osteoprotegerin/receptor activator of nuclear factor kappa-B ligand (OPG/RANKL) ratio in blood stream and the expression level of inflammatory markers in adipose tissues. Bone preservation by GPR40 stimulation is dependent on the presence of long-chain saturated fatty acids. CONCLUSION: GPR40 contributes to counter ovariectomy-induced bone loss in a context of saturated fatty acid enrichment.
G Protein-coupled Receptor 40 (GPR40) and Peroxisome Proliferator-activated Receptor gamma (PPARgamma): AN INTEGRATED TWO-RECEPTOR SIGNALING PATHWAY.[Pubmed:26105050]
J Biol Chem. 2015 Aug 7;290(32):19544-57.
Peroxisome proliferator-activated receptor gamma (PPARgamma) ligands have been widely used to treat type 2 diabetes mellitus. However, knowledge of PPARgamma signaling remains incomplete. In addition to PPARgamma, these drugs also activate G protein-coupled receptor 40 (GPR40), a Galphaq-coupled free fatty acid receptor linked to MAPK networks and glucose homeostasis. Notably, p38 MAPK activation has been implicated in PPARgamma signaling. Here, rosiglitazone (RGZ) activation of GPR40 and p38 MAPK was found to boost PPARgamma-induced gene transcription in human endothelium. Inhibition or knockdown of p38 MAPK or expression of a dominant negative (DN) p38 MAPK mutant blunted RGZ-induced PPARgamma DNA binding and reporter activity in EA.hy926 human endothelial cells. GPR40 inhibition or knockdown, or expression of a DN-Galphaq mutant likewise blocked activation of both p38 MAPK and PPARgamma reporters. Importantly, RGZ induction of PPARgamma target genes in primary human pulmonary artery endothelial cells (PAECs) was suppressed by knockdown of either p38 MAPK or GPR40. GPR40/PPARgamma signal transduction was dependent on p38 MAPK activation and induction of PPARgamma co-activator-1 (PGC1alpha). Silencing of p38 MAPK or GPR40 abolished the ability of RGZ to induce phosphorylation and expression of PGC1alpha in PAECs. Knockdown of PGC1alpha, its essential activator SIRT1, or its binding partner/co-activator EP300 inhibited RGZ induction of PPARgamma-regulated genes in PAECs. RGZ/GPR40/p38 MAPK signaling also led to EP300 phosphorylation, an event that enhances PPARgamma target gene transcription. Thus, GPR40 and PPARgamma can function as an integrated two-receptor signal transduction pathway, a finding with implications for rational drug development.
[Current status of clinical development of novel anti-diabetic drugs].[Pubmed:25812383]
Nihon Rinsho. 2015 Mar;73(3):517-22.
Clinical development of novel antidiabetic drugs, such as GK activator, GPR40 agonist, GPR119 agonist, 11beta-HSD1 inhibitor and trelagliptin, has been progressing over the world. Especially, GK activator, GPR40 agonist, GPR119 agonist and 112-HSD1 inhibitor have unique action mechanism compared to existing drugs. GK activator potentiates glucose-stimulated insulin secretion from beta cells and stimulates glucose uptake into the liver. GPR40 agonists and GPR119 agonist stimulate glucose-dependent insulin secretion from beta cells. 11beta-HSD1 inhibitor reduces the conversion of cortisone to cortisol. Trelagliptin is a long acting dipeptidyl peptidase-4(DPP-4) inhibitor and a once-weekly treatment by trelagliptin would improve the drug adherence of patients. This article focuses on these new and emerging diabetes agents.
Discovery of Chromane Propionic Acid Analogues as Selective Agonists of GPR120 with in Vivo Activity in Rodents.[Pubmed:28105282]
ACS Med Chem Lett. 2016 Dec 6;8(1):96-101.
GPR120 (FFAR4) is a fatty acid sensing G protein coupled receptor (GPCR) that has been identified as a target for possible treatment of type 2 diabetes. A selective activator of GPR120 containing a chromane scaffold has been designed, synthesized, and evaluated in vivo. Results of these efforts suggest that chromane propionic acid 18 is a suitable tool molecule for further animal studies. Compound 18 is selective over the closely related target GPR40 (FFAR1), has a clean off-target profile, demonstrates suitable pharmacokinetic properties, and has been evaluated in wild-type/knockout GPR120 mouse oGTT studies.
PPARgamma signaling and emerging opportunities for improved therapeutics.[Pubmed:27268145]
Pharmacol Res. 2016 Sep;111:76-85.
Peroxisome proliferator-activated receptor gamma (PPARgamma) is a ligand-activated nuclear receptor that regulates glucose and lipid metabolism, endothelial function and inflammation. Rosiglitazone (RGZ) and other thiazolidinedione (TZD) synthetic ligands of PPARgamma are insulin sensitizers that have been used for the treatment of type 2 diabetes. However, undesirable side effects including weight gain, fluid retention, bone loss, congestive heart failure, and a possible increased risk of myocardial infarction and bladder cancer, have limited the use of TZDs. Therefore, there is a need to better understand PPARgamma signaling and to develop safer and more effective PPARgamma-directed therapeutics. In addition to PPARgamma itself, many PPARgamma ligands including TZDs bind to and activate G protein-coupled receptor 40 (GPR40), also known as free fatty acid receptor 1. GPR40 signaling activates stress kinase pathways that ultimately regulate downstream PPARgamma responses. Recent studies in human endothelial cells have demonstrated that RGZ activation of GPR40 is essential to the optimal propagation of PPARgamma genomic signaling. RGZ/GPR40/p38 MAPK signaling induces and activates PPARgamma co-activator-1alpha, and recruits E1A binding protein p300 to the promoters of target genes, markedly enhancing PPARgamma-dependent transcription. Therefore in endothelium, GPR40 and PPARgamma function as an integrated signaling pathway. However, GPR40 can also activate ERK1/2, a proinflammatory kinase that directly phosphorylates and inactivates PPARgamma. Thus the role of GPR40 in PPARgamma signaling may have important implications for drug development. Ligands that strongly activate PPARgamma, but do not bind to or activate GPR40 may be safer than currently approved PPARgamma agonists. Alternatively, biased GPR40 agonists might be sought that activate both p38 MAPK and PPARgamma, but not ERK1/2, avoiding its harmful effects on PPARgamma signaling, insulin resistance and inflammation. Such next generation drugs might be useful in treating not only type 2 diabetes, but also diverse chronic and acute forms of vascular inflammation such as atherosclerosis and septic shock.


