PimpinellinCAS# 131-12-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 131-12-4 | SDF | Download SDF |
| PubChem ID | 4825 | Appearance | Yellow powder |
| Formula | C13H10O5 | M.Wt | 246.2 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
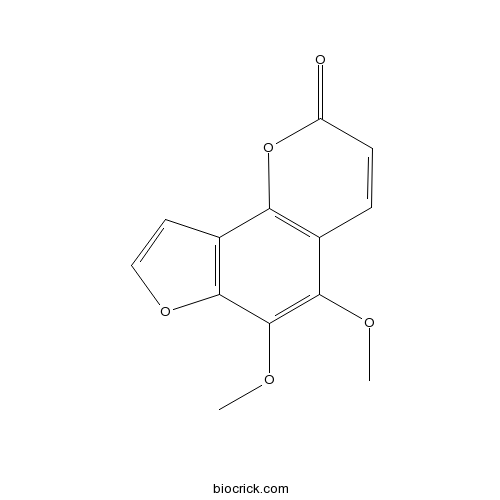
| Chemical Name | 5,6-dimethoxyfuro[2,3-h]chromen-2-one | ||
| SMILES | COC1=C(C2=C(C=CO2)C3=C1C=CC(=O)O3)OC | ||
| Standard InChIKey | BQPRWZCEKZLBHL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H10O5/c1-15-11-7-3-4-9(14)18-10(7)8-5-6-17-12(8)13(11)16-2/h3-6H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pimpinellin has phototoxic, it acts as antagonist of proteins with GABA receptor activity. Pimpinellin has anti- inflammatory, and cytotoxic activities. Pimpinellin also has anti-bacterial activity, it shows inhibitory activity against Mycobacterium tuberculosis strain H37Ra , with MICs of 812 uM and IC50s of 389 uM. |
| Targets | Antifection |
| In vitro | The Canadian medicinal plant Heracleum maximum contains antimycobacterial diynes and furanocoumarins.[Pubmed: 23501157]J Ethnopharmacol. 2013 May 2;147(1):232-7.Heracleum maximum is amongst the most commonly used plants by the indigenous peoples of North America. The First Nations of the eastern Canada use infusions of Heracleum maximum roots for the treatment of respiratory ailments including tuberculosis. Previous investigations of extracts derived from the roots of Heracleum maximum have shown it to possess antimycobacterial activity.
To isolate and identify antimycobacterial constituents from the roots of Heracleum maximum.
|
| In vivo | Simultaneous determination of pimpinellin, isopimpinellin and phellopterin in rat plasma by a validated UPLC-MS/MS and its application to a pharmacokinetic study after administration of Toddalia asiatica extract.[Pubmed: 22418072]J Chromatogr B Analyt Technol Biomed Life Sci. 2012 Apr 1;891-892:102-8.
|
| Cell Research | Discovery and antitumor activities of constituents from Cyrtomium fortumei (J.) Smith rhizomes.[Pubmed: 23379693 ]Chem Cent J. 2013 Feb 4;7(1):24.Cyrtomium fortumei (J.) Smith is an important Chinese herbal medicine because of its biological functions. However, systematic and comprehensive studies on the phytochemicals from Cyrtomium fortumei (J.) Smith and their bioactivity are limited.
|
| Structure Identification | Contact Dermatitis. 1983 Sep;9(5):364-6.Phototoxicity from furocoumarins (psoralens) of Heracleum laciniatum in a patient with vitiligo. Action spectrum studies on bergapten, pimpinellin, angelicin and sphondin.[Pubmed: 6627920]Investigations on light reactions in a patient with vitiligo are presented. |

Pimpinellin Dilution Calculator

Pimpinellin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0617 mL | 20.3087 mL | 40.6174 mL | 81.2348 mL | 101.5435 mL |
| 5 mM | 0.8123 mL | 4.0617 mL | 8.1235 mL | 16.247 mL | 20.3087 mL |
| 10 mM | 0.4062 mL | 2.0309 mL | 4.0617 mL | 8.1235 mL | 10.1543 mL |
| 50 mM | 0.0812 mL | 0.4062 mL | 0.8123 mL | 1.6247 mL | 2.0309 mL |
| 100 mM | 0.0406 mL | 0.2031 mL | 0.4062 mL | 0.8123 mL | 1.0154 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dimethyl phthalate
Catalog No.:BCN6167
CAS No.:131-11-3
- alpha-Yohimbine
Catalog No.:BCN6166
CAS No.:131-03-3
- VU 0360172 hydrochloride
Catalog No.:BCC6141
CAS No.:1309976-62-2
- 15-Methoxymkapwanin
Catalog No.:BCN6498
CAS No.:1309920-99-7
- MRS 4062 triethylammonium salt
Catalog No.:BCC6134
CAS No.:1309871-50-8
- Sephin1
Catalog No.:BCC3980
CAS No.:13098-73-2
- (±)-CPSI 1306
Catalog No.:BCC6161
CAS No.:1309793-47-2
- H 89 2HCl
Catalog No.:BCC4997
CAS No.:130964-39-5
- Marumoside A
Catalog No.:BCN7702
CAS No.:1309604-34-9
- Wittifuran X
Catalog No.:BCN4794
CAS No.:1309478-07-6
- K145
Catalog No.:BCC4305
CAS No.:1309444-75-4
- GPR40 Activator 1
Catalog No.:BCC4125
CAS No.:1309435-60-6
- N-Acetylneuraminic acid
Catalog No.:BCN2204
CAS No.:131-48-6
- Oxybenzone
Catalog No.:BCC5445
CAS No.:131-57-7
- Meptyldinocap
Catalog No.:BCC5468
CAS No.:131-72-6
- 8-Epiloganic acid
Catalog No.:BCC8956
CAS No.:82509-41-9
- Boldenone undecylenate
Catalog No.:BCC8896
CAS No.:13103-34-9
- GNF179 Metabolite
Catalog No.:BCC5176
CAS No.:1310455-86-7
- NB-598
Catalog No.:BCC1786
CAS No.:131060-14-5
- Tubastatin A HCl
Catalog No.:BCC3877
CAS No.:1310693-92-5
- NVP-BGJ398 phosphate
Catalog No.:BCC1814
CAS No.:1310746-10-1
- Miltipolone
Catalog No.:BCN3222
CAS No.:131086-61-8
- 5,7-Dichlorokynurenic acid
Catalog No.:BCC6592
CAS No.:131123-76-7
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
The Canadian medicinal plant Heracleum maximum contains antimycobacterial diynes and furanocoumarins.[Pubmed:23501157]
J Ethnopharmacol. 2013 May 2;147(1):232-7.
ETHNOPHARMACOLOGICAL RELEVANCE: Heracleum maximum is amongst the most commonly used plants by the indigenous peoples of North America. The First Nations of the eastern Canada use infusions of Heracleum maximum roots for the treatment of respiratory ailments including tuberculosis. Previous investigations of extracts derived from the roots of Heracleum maximum have shown it to possess antimycobacterial activity. AIM OF THE STUDY: To isolate and identify antimycobacterial constituents from the roots of Heracleum maximum. MATERIALS AND METHODS: A methanolic extract of Heracleum maximum roots was subjected to bioassay guided fractionation using the microplate resazurin assay (MRA) to assess inhibitory activity against Mycobacterium tuberculosis strain H37Ra. The antimycobacterial constituents were identified by NMR, MS and polarimetry. RESULTS: The polyacetylene (3R,8S)-falcarindiol and the furanocoumarins bergapten, isobergapten, angelicin, sphondin, Pimpinellin, isoPimpinellin and 6-isopentenyloxyisobergapten were isolated from the Heracleum maximum root extract. (3R,8S)-Falcarindiol and 6-isopentenyloxyisobergapten exhibited MICs of 24 muM and 167 muM and IC50s of 6 muM and 27 muM against Mycobacterium tuberculosis H37Ra respectively. The remaining furanocoumarins bergapten, isobergapten, angelicin, sphondin, Pimpinellin, and isoPimpinellin were less active, with MICs of 925, 1850, 2149, 1859, 812 and 1625 muM and IC50s of 125, 344, 350, 351, 389 and 406 muM. CONCLUSIONS: (3R,8S)-Falcarindiol, bergapten, isobergapten, angelicin, sphondin, Pimpinellin, isoPimpinellin and 6-isopentenyloxyisobergapten were identified as the principal constituents responsible for the antimycobacterial activity of the roots of Heracleum maximum. This work supports the ethnopharmacological use of Heracleum maximum by Canadian First Nations and Native American communities as a treatment for infectious diseases, specifically tuberculosis.
Simultaneous determination of pimpinellin, isopimpinellin and phellopterin in rat plasma by a validated UPLC-MS/MS and its application to a pharmacokinetic study after administration of Toddalia asiatica extract.[Pubmed:22418072]
J Chromatogr B Analyt Technol Biomed Life Sci. 2012 Apr 1;891-892:102-8.
A rapid and selective ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method was developed for simultaneous determination of three bioactive coumarins of Toddalia asiatica extract including Pimpinellin, isoPimpinellin and phellopterin in rat plasma for the first time. Phenacetin was used as the internal standard (IS). Plasma samples were extracted by liquid-liquid extraction with methyl tert-butyl ether. The chromatographic separation was carried out on an ACQUITY UPLC BEH C(1)(8) column with an isocratic mobile phase consisting of methanol-5 mmol/L ammonium acetate (65:35, v/v). The detection was performed on a triple quadrupole tandem mass spectrometer by multiple reaction monitoring (MRM) via electrospray ionization (ESI) source with positive ionization mode. The method was linear for all analytes over investigated range with all correlation coefficients greater than 0.9942. The lower limits of quantification (LLOQ) were 25.0 ng/mL for Pimpinellin, 10.0 ng/mL for isoPimpinellin and 5.00 ng/mL for phellopterin. The intra- and inter-day precision (RSD%) was within 12% and the accuracy (RE%) ranged from -2.3% to 5.5%. The rapid and sensitive method was fully validated and successfully applied to the pharmacokinetic study of Pimpinellin, isoPimpinellin and phellopterin in rats following oral administration of Toddalia asiatica extract.
Discovery and antitumor activities of constituents from Cyrtomium fortumei (J.) Smith rhizomes.[Pubmed:23379693]
Chem Cent J. 2013 Feb 4;7(1):24.
UNLABELLED: BACKGROUND: Cyrtomium fortumei (J.) Smith is an important Chinese herbal medicine because of its biological functions. However, systematic and comprehensive studies on the phytochemicals from Cyrtomium fortumei (J.) Smith and their bioactivity are limited. RESULTS: Using the bioassay-guided technique, the ethyl acetate and n-BuOH extracts of the rhizomes of Cyrtomium fortumei (J.) Smith were shown to exhibit good antitumor activities, consequently leading to the isolation of 23 compounds. All compounds were isolated from the plant for the first time. The inhibitory activities of these compounds were investigated on tumor cells MGC-803, PC3, and A375 in vitro by MTT (thiazolyl blue tetrazolium bromide) assay, and the results showed that Pimpinellin (3) had potent cytotoxic activities against the three cell lines, with the IC50 values of 14.4 +/- 0.3 muM, 20.4 +/- 0.5 muM, and 29.2 +/- 0.6 muM, respectively. The mechanism of the antitumor action indicated that Pimpinellin inhibited the growth of MGC-803 cells via the induction of tumor cell apoptosis, with apoptosis ratio of 27.44% after 72 h of treatment at 20 muM. CONCLUSIONS: This study suggests that most of the compounds from the roots of Cyrtomium fortumei (J.) Smith could inhibit the growth of human carcinoma cells. Moreover, Pimpinellin inhibited the growth of tumor cells via the induction of tumor cell apoptosis.
Phototoxicity from furocoumarins (psoralens) of Heracleum laciniatum in a patient with vitiligo. Action spectrum studies on bergapten, pimpinellin, angelicin and sphondin.[Pubmed:6627920]
Contact Dermatitis. 1983 Sep;9(5):364-6.
Investigations on light reactions in a patient with vitiligo are presented. The minimal erythema dose (MED) in the UVB area was approximately 1/3 of that in persons of skin type II. The application of furocoumarins (psoralens) increased light tolerance by 1 MED at 300-310 nm. Action spectrum studies with furocoumarins from Heracleum laciniatum showed the following order of potency: bergapten, Pimpinellin, angelicin and sphondin. The efficacy was highest at 325-350 nm, with maxima at 330-335 nm. Pimpinellin was recently found to be phototoxic, but an action spectrum of sphondin is reported for the first time.


