NB-598SE inhibitor CAS# 131060-14-5 |
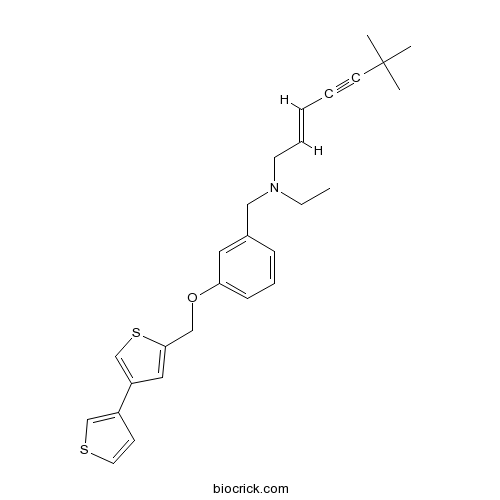
- NB-598
Catalog No.:BCC1786
CAS No.:131060-14-5
- NB-598 Maleate
Catalog No.:BCC1788
CAS No.:155294-62-5
- Terbinafine HCl
Catalog No.:BCC4863
CAS No.:78628-80-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 131060-14-5 | SDF | Download SDF |
| PubChem ID | 6443223 | Appearance | Powder |
| Formula | C27H31NOS2 | M.Wt | 449.67 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO | ||
| Chemical Name | (E)-N-ethyl-6,6-dimethyl-N-[[3-[(4-thiophen-3-ylthiophen-2-yl)methoxy]phenyl]methyl]hept-2-en-4-yn-1-amine | ||
| SMILES | CCN(CC=CC#CC(C)(C)C)CC1=CC(=CC=C1)OCC2=CC(=CS2)C3=CSC=C3 | ||
| Standard InChIKey | KIRGLCXNEVICOG-SOFGYWHQSA-N | ||
| Standard InChI | InChI=1S/C27H31NOS2/c1-5-28(14-8-6-7-13-27(2,3)4)18-22-10-9-11-25(16-22)29-19-26-17-24(21-31-26)23-12-15-30-20-23/h6,8-12,15-17,20-21H,5,14,18-19H2,1-4H3/b8-6+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | NB-598 is a potent and competitive inhibitor of squalene epoxidase (SE), and suppresses triglyceride biosynthesis through the farnesol pathway.In Vitro:NB598 (10 μM) causes a 36±7% reduction in total cholesterol level of MIN6 cells. NB598 causes a significant decrease in cholesterol by 49±2%, 46±7%, and 48±2% from PM, ER, and SG, respectively. NB598 dose-dependently inhibits insulin secretion under both basal (1 mM glucose) and glucose-stimulated (16.7 mM glucose) conditions. NB598 at concentrations up to 10 μM does not affect peak outward KV currents or the voltage dependence of activation but increases current inactivation[1]. NB-598 (10 μM) inhibits the synthesis of sterol and sterol ester from [14C]acetate without affecting the synthesis of other lipids such as phospholipids (PL), free fatty acids (FFA) and triacylglycerol (TG). In the absence of exogenous liposomal cholesterol, NB-598 reduces ACAT activity by 31%. NB-598 reduces ACAT activity by 22% even in the presence of a 600 PM concentration of liposomal cholesterol[2]. NB-598 suppresses the secretion of cholesterol and triacylglycerol from HepG2 cells into the medium[3]. References: | |||||

NB-598 Dilution Calculator

NB-598 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2239 mL | 11.1193 mL | 22.2385 mL | 44.4771 mL | 55.5963 mL |
| 5 mM | 0.4448 mL | 2.2239 mL | 4.4477 mL | 8.8954 mL | 11.1193 mL |
| 10 mM | 0.2224 mL | 1.1119 mL | 2.2239 mL | 4.4477 mL | 5.5596 mL |
| 50 mM | 0.0445 mL | 0.2224 mL | 0.4448 mL | 0.8895 mL | 1.1119 mL |
| 100 mM | 0.0222 mL | 0.1112 mL | 0.2224 mL | 0.4448 mL | 0.556 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
NB-598 is a potent competitive inhibitor of squalene epoxidase (SE). NB-598 suppresses triglyceride biosynthesis through the farnesol pathway. SE inhibitor NB-598 significantly inhibited both basal and glucose-stimulated insulin secretion from mouse pancreatic islets. CaV channels were markedly inhibited by NB-598.
- GNF179 Metabolite
Catalog No.:BCC5176
CAS No.:1310455-86-7
- Boldenone undecylenate
Catalog No.:BCC8896
CAS No.:13103-34-9
- 8-Epiloganic acid
Catalog No.:BCC8956
CAS No.:82509-41-9
- Meptyldinocap
Catalog No.:BCC5468
CAS No.:131-72-6
- Oxybenzone
Catalog No.:BCC5445
CAS No.:131-57-7
- N-Acetylneuraminic acid
Catalog No.:BCN2204
CAS No.:131-48-6
- Pimpinellin
Catalog No.:BCN6168
CAS No.:131-12-4
- Dimethyl phthalate
Catalog No.:BCN6167
CAS No.:131-11-3
- alpha-Yohimbine
Catalog No.:BCN6166
CAS No.:131-03-3
- VU 0360172 hydrochloride
Catalog No.:BCC6141
CAS No.:1309976-62-2
- 15-Methoxymkapwanin
Catalog No.:BCN6498
CAS No.:1309920-99-7
- MRS 4062 triethylammonium salt
Catalog No.:BCC6134
CAS No.:1309871-50-8
- Tubastatin A HCl
Catalog No.:BCC3877
CAS No.:1310693-92-5
- NVP-BGJ398 phosphate
Catalog No.:BCC1814
CAS No.:1310746-10-1
- Miltipolone
Catalog No.:BCN3222
CAS No.:131086-61-8
- 5,7-Dichlorokynurenic acid
Catalog No.:BCC6592
CAS No.:131123-76-7
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- mGlu2 agonist
Catalog No.:BCC1745
CAS No.:1311385-32-6
- Sodium Demethylcantharidate
Catalog No.:BCN8394
CAS No.:13114-29-9
- NSC 624206
Catalog No.:BCC7988
CAS No.:13116-77-3
- Cornuside
Catalog No.:BCN5007
CAS No.:131189-57-6
- PF 5081090
Catalog No.:BCC6148
CAS No.:1312473-63-4
- Antibiotic PF 1018
Catalog No.:BCN2149
CAS No.:131256-42-3
- Scutebarbatine W
Catalog No.:BCN7011
CAS No.:1312716-25-8
Effects of NB-598, a potent squalene epoxidase inhibitor, on the apical membrane uptake of cholesterol and basolateral membrane secretion of lipids in Caco-2 cells.[Pubmed:8347152]
Biochem Pharmacol. 1993 Jul 20;46(2):297-305.
Caco-2 cells grown on membrane filters were used as a model to study the effects of NB-598, an inhibitor of squalene epoxidase, on cholesterol absorption from the intestinal epithelia. NB-598 (10 microM) inhibited the synthesis of sterol and sterol ester from [14C]acetate without affecting the synthesis of other lipids such as phospholipids (PL), free fatty acids (FFA) and triacylglycerol (TG). When labeled lipid was apically loaded as a micellar lipid solution into Caco-2 cell cultures, NB-598 reduced basolaterally secreted radioactivity in cholesterol, cholesterol ester, PL and TG. Furthermore, NB-598 suppressed the basolateral secretion of apolipoprotein (apo) B. When microsomes prepared from control Caco-2 cells were incubated with 10 microM NB-598, acyl CoA:cholesterol acyltransferase (ACAT) activity was inhibited slightly. After incubating Caco-2 cells with 10 microM NB-598, a slight reduction in cellular ACAT activity was also observed. These results suggest that suppression of the secretion of particles containing apo B and reduction of cellular ACAT activity in the intestinal epithelia are part of the mechanism of the cholesterol-lowering effect of NB-598.
Effect of an inhibitor of squalene epoxidase, NB-598, on lipid metabolism in Hep G2 cells.[Pubmed:1606641]
Chem Pharm Bull (Tokyo). 1992 Feb;40(2):436-40.
NB-598, a potent inhibitor of squalene epoxidase, inhibited cholesterol synthesis from [14C]acetate and induced intracellular squalene accumulation in Hep G2 cells. NB-598 inhibited cholesterol synthesis from [14C]acetate, [3H]mevalonate, and [3H]squalene, but not from [3H]2,3-oxidosqualene in Hep G2 cells. It reduced cholesterol ester synthesis remarkably in the absence of exogenous cholesterol. This compound did not have any effect on the synthesis of ubiquinone and dolichol. When Hep G2 cells were prelabeled with micellar [3H]cholesterol, NB-598 did not affect the excretion of bile acid incorporated from [3H]cholesterol. However, NB-598 decreased the secretion of free and esterified cholesterol, triacylglycerol, and phospholipids, and increased the secretion of squalene. NB-598 is thought not only to inhibit cholesterol synthesis, but also to inhibit the secretion of lipids.
An inhibitor of squalene epoxidase, NB-598, suppresses the secretion of cholesterol and triacylglycerol and simultaneously reduces apolipoprotein B in HepG2 cells.[Pubmed:8504141]
Biochim Biophys Acta. 1993 May 20;1168(1):45-51.
NB-598, a specific inhibitor of squalene epoxidase, suppressed the secretion of cholesterol and triacylglycerol from HepG2 cells into the medium. L-654,969, an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, inhibited the secretion of cholesterol as potently as NB-598, but did not suppress the secretion of triacylglycerol. Both compounds decreased the intracellular cholesterol content almost equally, and neither of the compounds reduced the intracellular triacylglycerol content. The suppression of lipid secretion by NB-598 was associated with a significant reduction in apolipoprotein (apo) B secretion into the medium. Therefore, the suppression of lipid secretion by NB-598 may be caused by a reduction in the number of triacylglycerol-rich lipoprotein particles. In contrast, the suppression of cholesterol secretion by L-654,969 may be due to a modulation of lipoprotein lipid composition, since this agent did not reduce the secretion of apo B or triacylglycerol. The secretion of apo A-I was unaffected by either NB-598 or L-654,969. Pulse chase studies using [35S]methionine showed that the suppression of apo B secretion by NB-598 depended on an enhancement of intracellular degradation of apo B. These results indicate that the secretion of apo B from HepG2 cells is not regulated by the lipid synthesis alone, and suggest that the mechanism of the hypolipidemic effect of NB-598 involves the suppression of triacylglycerol-rich lipoprotein secretion from the liver as well as an inhibition of cholesterol synthesis in the liver.
Effect of a novel squalene epoxidase inhibitor, NB-598, on the regulation of cholesterol metabolism in Hep G2 cells.[Pubmed:1649182]
J Biol Chem. 1991 Jul 15;266(20):13171-7.
We have reported previously that NB-598 competitively inhibits human squalene epoxidase and strongly inhibits cholesterol synthesis from [14C]acetate in cultured cells. Furthermore, multiple oral administration of NB-598 decreased serum cholesterol levels in dogs (Horie, M., Tsuchiya, Y., Hayashi, M., Iida, Y., Iwasawa, Y., Nagata, Y., Sawasaki, Y., Fukuzumi, H., Kitani, K., and Kamei, T. (1990) J. Biol. Chem. 265, 18075-18078). In the present study, the effects of NB-598 on 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase and low-density-lipoprotein (LDL) receptor were examined using a human hepatoma cell line Hep G2. Incubation of Hep G2 cells with NB-598 for 18 h increased HMG-CoA reductase activity in a dose-dependent manner. However, the increase in activity induced by NB-598 was lower than that induced by L-654,969 (a potent HMG-CoA reductase inhibitor), although NB-598 inhibited cholesterol synthesis more potently than L-654,969. On the other hand, HMG-CoA reductase mRNA was increased to the same extent by both inhibitors. These results demonstrate that NB-598 does not inhibit the synthesis of non-sterol derivative(s) of mevalonate, which regulate HMG-CoA reductase activity at the post-transcriptional level. NB-598 increased the binding of 125I-LDL to Hep G2 cells. LDL receptor mRNA was also induced by NB-598. In the presence of LDL or cycloheximide, NB-598 did not increase LDL receptor activity. These results demonstrate that the induction of LDL receptor activity by NB-598 is due to increases in mRNA and protein through the inhibition of cholesterol synthesis at the squalene epoxidase step. From these observations, squalene epoxidase inhibitor is expected to be highly effective in the treatment of hypercholesterolemia and also is very useful as a research tool for studying the regulation of cholesterol metabolism.


