PF 5081090CAS# 1312473-63-4 |
- LY2606368
Catalog No.:BCC4105
CAS No.:1234015-52-1
- CHIR-124
Catalog No.:BCC3750
CAS No.:405168-58-3
- BML-277
Catalog No.:BCC4245
CAS No.:516480-79-8
- AZD7762
Catalog No.:BCC2555
CAS No.:860352-01-8
- MK-8776 (SCH-900776)
Catalog No.:BCC3817
CAS No.:891494-63-6
- SCH900776 S-isomer
Catalog No.:BCC1936
CAS No.:891494-64-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

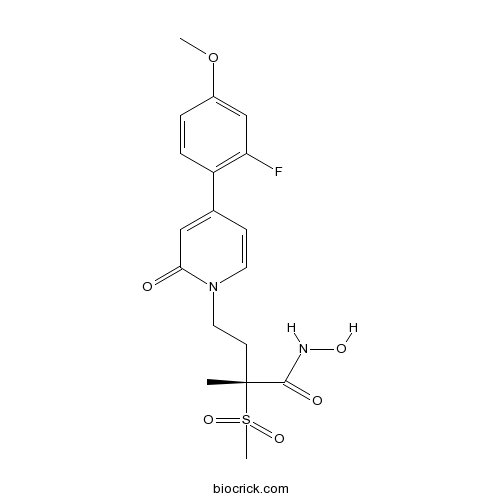
| Cas No. | 1312473-63-4 | SDF | Download SDF |
| PubChem ID | 56964907 | Appearance | Powder |
| Formula | C18H21FN2O6S | M.Wt | 412.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
| Chemical Name | (2R)-4-[4-(2-fluoro-4-methoxyphenyl)-2-oxopyridin-1-yl]-N-hydroxy-2-methyl-2-methylsulfonylbutanamide | ||
| SMILES | CC(CCN1C=CC(=CC1=O)C2=C(C=C(C=C2)OC)F)(C(=O)NO)S(=O)(=O)C | ||
| Standard InChIKey | DNVUWHWBCMGQLU-GOSISDBHSA-N | ||
| Standard InChI | InChI=1S/C18H21FN2O6S/c1-18(17(23)20-24,28(3,25)26)7-9-21-8-6-12(10-16(21)22)14-5-4-13(27-2)11-15(14)19/h4-6,8,10-11,24H,7,9H2,1-3H3,(H,20,23)/t18-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent antibacterial agent which disrupts lipid bilayer synthesis through inhibition of LpxC (IC50 = 1.1 nM in Pseudomonas aeruginosa); effective against a range of gram negative bacteria. |

PF 5081090 Dilution Calculator

PF 5081090 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4247 mL | 12.1233 mL | 24.2465 mL | 48.4931 mL | 60.6163 mL |
| 5 mM | 0.4849 mL | 2.4247 mL | 4.8493 mL | 9.6986 mL | 12.1233 mL |
| 10 mM | 0.2425 mL | 1.2123 mL | 2.4247 mL | 4.8493 mL | 6.0616 mL |
| 50 mM | 0.0485 mL | 0.2425 mL | 0.4849 mL | 0.9699 mL | 1.2123 mL |
| 100 mM | 0.0242 mL | 0.1212 mL | 0.2425 mL | 0.4849 mL | 0.6062 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cornuside
Catalog No.:BCN5007
CAS No.:131189-57-6
- NSC 624206
Catalog No.:BCC7988
CAS No.:13116-77-3
- Sodium Demethylcantharidate
Catalog No.:BCN8394
CAS No.:13114-29-9
- mGlu2 agonist
Catalog No.:BCC1745
CAS No.:1311385-32-6
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- 5,7-Dichlorokynurenic acid
Catalog No.:BCC6592
CAS No.:131123-76-7
- Miltipolone
Catalog No.:BCN3222
CAS No.:131086-61-8
- NVP-BGJ398 phosphate
Catalog No.:BCC1814
CAS No.:1310746-10-1
- Tubastatin A HCl
Catalog No.:BCC3877
CAS No.:1310693-92-5
- NB-598
Catalog No.:BCC1786
CAS No.:131060-14-5
- GNF179 Metabolite
Catalog No.:BCC5176
CAS No.:1310455-86-7
- Boldenone undecylenate
Catalog No.:BCC8896
CAS No.:13103-34-9
- Antibiotic PF 1018
Catalog No.:BCN2149
CAS No.:131256-42-3
- Scutebarbatine W
Catalog No.:BCN7011
CAS No.:1312716-25-8
- Scutebarbatine X
Catalog No.:BCN6997
CAS No.:1312716-26-9
- Scutebarbatine Y
Catalog No.:BCN6994
CAS No.:1312716-27-0
- Scutebarbatine Z
Catalog No.:BCN6991
CAS No.:1312716-28-1
- Ro 25-6981 Maleate
Catalog No.:BCC4159
CAS No.:1312991-76-6
- Ro 8-4304 hydrochloride
Catalog No.:BCC7655
CAS No.:1312991-77-7
- threo Ifenprodil hemitartrate
Catalog No.:BCC7508
CAS No.:1312991-83-5
- Nystose
Catalog No.:BCN5397
CAS No.:13133-07-8
- NVP-CGM097
Catalog No.:BCC5395
CAS No.:1313363-54-0
- erythro-Guaiacylglycerol beta-threo-syringylglycerol ether
Catalog No.:BCN7333
CAS No.:1313434-74-0
- Periplocin
Catalog No.:BCN2655
CAS No.:13137-64-9
Improved disease activity with fosdagrocorat (PF-04171327), a partial agonist of the glucocorticoid receptor, in patients with rheumatoid arthritis: a Phase 2 randomized study.[Pubmed:28328159]
Int J Rheum Dis. 2017 Aug;20(8):960-970.
AIM: To assess efficacy and safety of fosdagrocorat (PF-04171327), a potential dissociated agonist of the glucocorticoid receptor, in rheumatoid arthritis (RA) patients. METHODS: This multicenter, double-blind, parallel-group, active- and placebo-controlled Phase 2 study (NCT00938587) randomized 86 patients (1 : 1 : 1 : 1) to receive fosdagrocorat 10 mg, fosdagrocorat 25 mg, prednisone 5 mg or placebo, all with stable background methotrexate therapy. The primary outcome was change from baseline in Disease Activity Score of 28 joints (DAS28-4[C-reactive protein (CRP)]) after 2 weeks of treatment. Secondary outcomes included American College of Rheumatology (ACR) response rates, change from baseline in ACR core components and Health Assessment Questionnaire Disability Index. RESULTS: At week 2, improvements from baseline in DAS28-4(CRP) with fosdagrocorat 10 and 25 mg, prednisone 5 mg and placebo were -1.69, -2.22, -1.17 and -0.96, respectively, and were statistically significantly greater for both fosdagrocorat doses versus placebo (P < 0.05) and for fosdagrocorat 25 mg versus prednisone 5 mg (P < 0.001). The effects of fosdagrocorat on secondary outcomes were generally consistent with those observed for the primary outcome. Adverse events (AEs) were reported for eight (38%), three (14%), four (19%) and 12 (55%) patients treated with fosdagrocorat 10 and 25 mg, prednisone 5 mg and placebo, respectively. Most AEs were mild in severity. Four patients discontinued treatment due to AEs (fosdagrocorat 10 mg, n = 2; placebo, n = 2). There were no serious AEs. CONCLUSION: Fosdagrocorat 10 and 25 mg demonstrated efficacy in improving signs and symptoms in RA patients, with manageable AEs. Additional studies are needed to assess the longer-term safety and efficacy of fosdagrocorat.
Clinical Activity of the gamma-Secretase Inhibitor PF-03084014 in Adults With Desmoid Tumors (Aggressive Fibromatosis).[Pubmed:28350521]
J Clin Oncol. 2017 May 10;35(14):1561-1569.
Purpose Desmoid tumors (aggressive fibromatosis) arise from connective tissue cells or fibroblasts. In general, they are slow growing and do not metastasize; however, locally aggressive desmoid tumors can cause severe morbidity and loss of function. Disease recurrence after surgery and/or radiation and diagnosis of multifocal desmoid tumors highlight the need to develop effective systemic treatments for this disease. In this study, we evaluate objective response rate after therapy with the gamma-secretase inhibitor PF-03084014 in patients with recurrent, refractory, progressive desmoid tumors. Patients and Methods Seventeen patients with desmoid tumors received PF-03084014 150 mg orally twice a day in 3-week cycles. Response to treatment was evaluated at cycle 1 and every six cycles, that is, 18 weeks, by RECIST (Response Evaluation Criteria in Solid Tumors) version 1.1. Patient-reported outcomes were measured at baseline and at every restaging visit by using the MD Anderson Symptoms Inventory. Archival tumor and blood samples were genotyped for somatic and germline mutations in APC and CTNNB1. Results Of 17 patients accrued to the study, 15 had mutations in APC or CTNNB1 genes. Sixteen patients (94%) were evaluable for response; five (29%) experienced a confirmed partial response and have been on study for more than 2 years. Another five patients with prolonged stable disease as their best response remain on study. Patient-reported outcomes confirmed clinician reporting that the investigational agent was well tolerated and, in subgroup analyses, participants who demonstrated partial response also experienced clinically meaningful and statistically significant improvements in symptom burden. Conclusion PF-03084014 was well tolerated and demonstrated promising clinical benefit in patients with refractory, progressive desmoid tumors who receive long-term treatment.
Multireference configuration interaction study of the 27 low-lying states of the PF(+) cation.[Pubmed:28365453]
Spectrochim Acta A Mol Biomol Spectrosc. 2017 Jun 15;181:226-238.
This paper studied the spectroscopic parameters and vibrational properties of 27 Lambda-S and 60Omega states of PF(+) cation. The 27 Lambda-S states were the X(2)Pi, A(2)Sigma(+), B(2)Pi, C(2)Sigma(-), D(2)Delta, a(4)Sigma(-), b(4)Pi, c(4)Sigma(+), d(4)Delta, 2(2)Sigma(+), 3(2)Sigma(+), 4(2)Sigma(+), 2(2)Sigma(-), 3(2)Sigma(-), 3(2)Pi, 4(2)Pi, 5(2)Pi, 6(2)Pi, 2(2)Delta, 3(2)Delta, 1(2)Phi, 2(4)Sigma(-), 3(4)Sigma(-), 2(4)Pi, 3(4)Pi, 1(6)Sigma(-), and 1(6)Pi, which were generated from the first four dissociation limits. The 60Omega states were produced from the 27 Lambda-S states. All the potential energy curves were calculated with the CASSCF method, which was followed by the icMRCI+Q approach. The a(4)Sigma(-), b(4)Pi, and D(2)Delta states were inverted with the spin-orbit coupling effect accounted for. The 2(4)Pi, 2(4)Sigma(-), 2(2)Delta, 3(2)Delta, 3(2)Sigma(+), 4(2)Sigma(+), 1(2)Phi, and 2(2)Sigma(-) states were repulsive whether the spin-orbit coupling effect was included or not, but the 5(2)Pi and D(2)Delta states became repulsive only with the spin-orbit coupling effect included. The C(2)Sigma(-) state was very weakly bound. The a(4)Sigma(-) state had one barrier. The avoided crossings existed between the a(4)Sigma(-) and 2(4)Sigma(-) states, the 2(2)Sigma(+) and 3(2)Sigma(+) states as well as the D(2)Delta and 2(2)Delta states. Core-valence correlation and scalar relativistic corrections were taken into account. The extrapolation to the complete basis set limit was done. The spectroscopic parameters and vibrational properties were determined. The transition dipole moments were calculated and the Franck-Condon factors of some electric dipole transitions were evaluated. The spin-orbit coupling effect on the spectroscopic and vibrational properties was discussed.
Entrapment of DyP-type peroxidase from Pseudomonas fluorescens Pf-5 into Ca-alginate magnetic beads.[Pubmed:28326617]
Biotechnol Appl Biochem. 2018 Mar;65(2):238-245.
The aim of this study was to investigate the optimal conditions for the immobilization and stabilization of DyP1B dye decolorizing peroxidases type B (DyP1B) from Pseudomonas fluorescens Pf-5 immobilized in Ca-alginate ferromagnetic beads. The immobilized DyP1B was used in the degradation of the Reactive Blue 5 (RB5) synthetic dye. The enzyme was successfully entrapped in a Ca-alginate matrix and showed an encapsulation efficiency of 94%. The concentration of DyP1B (0.8 mg mL(-1) ), 2% of alginate and magnetite (10.0 mg mL(-1) ) was optimal for immobilization. The immobilized DyP1B showed optimum activity at pH 7.0 and 40 degrees C compared with pH 5.5 and 30 degrees C for free peroxidase. Reusability studies showed that after five cycles, the immobilized DyP1B system retained more than 58% of its initial activity. The immobilized DyP1B was able to decolorize RB5 at concentrations of 0.1, 0.05, and 0.01% (w v(-1) ) with efficiency rates of about 20, 29, and 45%, respectively. The immobilization of DyP1B in alginate beads with the addition of Fe3 O4 increased its catalytic and applicative potential.


