N-Acetylneuraminic acidCAS# 131-48-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 131-48-6 | SDF | Download SDF |
| PubChem ID | 906 | Appearance | White powder |
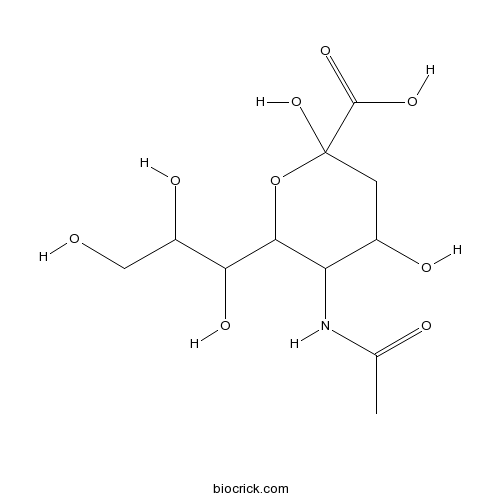
| Formula | C11H19NO9 | M.Wt | 309.27 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | NANA; Lactaminic acid | ||
| Solubility | H2O : ≥ 100 mg/mL (323.34 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 5-acetamido-2,4-dihydroxy-6-(1,2,3-trihydroxypropyl)oxane-2-carboxylic acid | ||
| SMILES | CC(=O)NC1C(CC(OC1C(C(CO)O)O)(C(=O)O)O)O | ||
| Standard InChIKey | SQVRNKJHWKZAKO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H19NO9/c1-4(14)12-7-5(15)2-11(20,10(18)19)21-9(7)8(17)6(16)3-13/h5-9,13,15-17,20H,2-3H2,1H3,(H,12,14)(H,18,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | N-Acetylneuraminic acid is a nine-carbon, sialic acid monosaccharide commonly found in glycoproteins on cell membranes and in glycolipids such as gangliosides in mammalian cells.Synthesis of pyrrolidine analogues of N‐acetylneuraminic acid could as potential sialidase inhibitors. N-Acetylneuraminic acid-containing substances has growth-promoting effects on bifidobacteria, a number of N-acetylneuraminic acid-based compounds as potential rotavirus inhibitors. |
| Targets | Antifection |
| In vitro | Aeromonas salmonicida binds differentially to mucins isolated from skin and intestinal regions of Atlantic salmon in an N-acetylneuraminic acid-dependent manner.[Pubmed: 25287918]Infect Immun. 2014 Dec;82(12):5235-45.Aeromonas salmonicida subsp. salmonicida infection, also known as furunculosis disease, is associated with high morbidity and mortality in salmonid aquaculture. The first line of defense the pathogen encounters is the mucus layer, which is predominantly comprised of secreted mucins. Synthesis and biological evaluation of N-acetylneuraminic acid-based rotavirus inhibitors.[Pubmed: 8632438]J Med Chem. 1996 Mar 15;39(6):1314-20.Rotavirus can cause severe gastrointestinal disease, especially in infants and young children, and is particularly prevalent in Third-World countries. Therefore, the development of potential inhibitors of this virus is of great interest. Growth-promoting Effects of N-Acetylneuraminic Acid-containing Substances on Bifidobacteria.[Reference: WebLink]Biosci. Biotech. Biochem. 1994, 58(9):1720-2.
|
| Structure Identification | Carbohydr Res. 2015 Jan 30;402:133-45.Characterization of the N-acetylneuraminic acid synthase (NeuB) from the psychrophilic fish pathogen Moritella viscosa.[Pubmed: 25498013]Moritella viscosa is a Gram-negative psychrophilic bacterium that causes winter ulcer disease in Atlantic salmon and cod. Its genome reveals that it possesses the ability to synthesize sialic acids. Indeed, sialic acid can be isolated from the bacterium and when analyzed using HPLC-MS/MS, the presence of N-Acetylneuraminic acid was confirmed. Thus, the N-Acetylneuraminic acid synthase NeuB from M. viscosa (MvNeuB) was recombinantly produced and characterized. |

N-Acetylneuraminic acid Dilution Calculator

N-Acetylneuraminic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2334 mL | 16.1671 mL | 32.3342 mL | 64.6684 mL | 80.8355 mL |
| 5 mM | 0.6467 mL | 3.2334 mL | 6.4668 mL | 12.9337 mL | 16.1671 mL |
| 10 mM | 0.3233 mL | 1.6167 mL | 3.2334 mL | 6.4668 mL | 8.0836 mL |
| 50 mM | 0.0647 mL | 0.3233 mL | 0.6467 mL | 1.2934 mL | 1.6167 mL |
| 100 mM | 0.0323 mL | 0.1617 mL | 0.3233 mL | 0.6467 mL | 0.8084 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
N-Acetylneuraminic acid is a nine-carbon, sialic acid monosaccharide commonly found in glycoproteins on cell membranes and in glycolipids such as gangliosides in mammalian cells. Studies suggest that N-Acetylneuraminic acid is useful biologically in neurotransmission, leukocyte extravasation, viral or bacterial infections and carbohydrate-protein recognition.
References:
[1]. Bondioli L et al. PLGA nanoparticles surface decorated with the sialic acid, N-acetylneuraminic acid. Biomaterials. 2010 Apr;31(12):3395-403.
[2]. Bondioli L et al. PLGA nanoparticles surface decorated with the sialic acid, N-acetylneuraminic acid. Biomaterials. 2010 Apr;31(12):3395-403.
- Pimpinellin
Catalog No.:BCN6168
CAS No.:131-12-4
- Dimethyl phthalate
Catalog No.:BCN6167
CAS No.:131-11-3
- alpha-Yohimbine
Catalog No.:BCN6166
CAS No.:131-03-3
- VU 0360172 hydrochloride
Catalog No.:BCC6141
CAS No.:1309976-62-2
- 15-Methoxymkapwanin
Catalog No.:BCN6498
CAS No.:1309920-99-7
- MRS 4062 triethylammonium salt
Catalog No.:BCC6134
CAS No.:1309871-50-8
- Sephin1
Catalog No.:BCC3980
CAS No.:13098-73-2
- (±)-CPSI 1306
Catalog No.:BCC6161
CAS No.:1309793-47-2
- H 89 2HCl
Catalog No.:BCC4997
CAS No.:130964-39-5
- Marumoside A
Catalog No.:BCN7702
CAS No.:1309604-34-9
- Wittifuran X
Catalog No.:BCN4794
CAS No.:1309478-07-6
- K145
Catalog No.:BCC4305
CAS No.:1309444-75-4
- Oxybenzone
Catalog No.:BCC5445
CAS No.:131-57-7
- Meptyldinocap
Catalog No.:BCC5468
CAS No.:131-72-6
- 8-Epiloganic acid
Catalog No.:BCC8956
CAS No.:82509-41-9
- Boldenone undecylenate
Catalog No.:BCC8896
CAS No.:13103-34-9
- GNF179 Metabolite
Catalog No.:BCC5176
CAS No.:1310455-86-7
- NB-598
Catalog No.:BCC1786
CAS No.:131060-14-5
- Tubastatin A HCl
Catalog No.:BCC3877
CAS No.:1310693-92-5
- NVP-BGJ398 phosphate
Catalog No.:BCC1814
CAS No.:1310746-10-1
- Miltipolone
Catalog No.:BCN3222
CAS No.:131086-61-8
- 5,7-Dichlorokynurenic acid
Catalog No.:BCC6592
CAS No.:131123-76-7
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- mGlu2 agonist
Catalog No.:BCC1745
CAS No.:1311385-32-6
Characterization of the N-acetylneuraminic acid synthase (NeuB) from the psychrophilic fish pathogen Moritella viscosa.[Pubmed:25498013]
Carbohydr Res. 2015 Jan 30;402:133-45.
Moritella viscosa is a Gram-negative psychrophilic bacterium that causes winter ulcer disease in Atlantic salmon and cod. Its genome reveals that it possesses the ability to synthesize sialic acids. Indeed, sialic acid can be isolated from the bacterium and when analyzed using HPLC-MS/MS, the presence of N-Acetylneuraminic acid was confirmed. Thus, the N-Acetylneuraminic acid synthase NeuB from M. viscosa (MvNeuB) was recombinantly produced and characterized. The optimum pH and temperature for MvNeuB activity are 7.5 and 30 degrees C, respectively. The KM for N-acetylmannosamine and phosphoenolpyruvate is 18+/-5 and 0.8+/-0.2 mM, respectively. The kcat value ( approximately 225 min(-1)) for both N-acetylmannosamine and phosphoenolpyruvate is the highest turnover number found for an enzyme in this class until the date. A calorimetric study of MvNeuB shows that the enzyme has a two-step transition peak probably reflecting the two domains these proteins consist of. MvNeuB is less stable at higher temperature and has a high catalytic activity at lower temperature compared to mesophilic counterparts. Enzymes from psychrophilic organisms are generally cold adapted meaning they can maintain adequate function near the freezing point of water. Cold adapted enzymes are catalytically more efficient at lower temperature and are more thermo-labile compared to their mesophilic counterparts. MvNeuB is a typical cold adapted enzyme and could be further explored for production of sialic acids and derivates at low temperatures.
Synthesis and biological evaluation of N-acetylneuraminic acid-based rotavirus inhibitors.[Pubmed:8632438]
J Med Chem. 1996 Mar 15;39(6):1314-20.
Rotavirus can cause severe gastrointestinal disease, especially in infants and young children, and is particularly prevalent in Third-World countries. Therefore, the development of potential inhibitors of this virus is of great interest. The present study describes the synthesis and in vitro biological evaluation of a number of N-Acetylneuraminic acid-based compounds as potential rotavirus inhibitors. Our data suggests that it is indeed possible to inhibit adhesion of the virus, and hence in vitro replication, with carbohydrate-based molecules, although this inhibition does appear to be strain dependent.
Aeromonas salmonicida binds differentially to mucins isolated from skin and intestinal regions of Atlantic salmon in an N-acetylneuraminic acid-dependent manner.[Pubmed:25287918]
Infect Immun. 2014 Dec;82(12):5235-45.
Aeromonas salmonicida subsp. salmonicida infection, also known as furunculosis disease, is associated with high morbidity and mortality in salmonid aquaculture. The first line of defense the pathogen encounters is the mucus layer, which is predominantly comprised of secreted mucins. Here we isolated and characterized mucins from the skin and intestinal tract of healthy Atlantic salmon and studied how A. salmonicida bound to them. The mucins from the skin, pyloric ceca, and proximal and distal intestine mainly consisted of mucins soluble in chaotropic agents. The mucin density and mucin glycan chain length from the skin were lower than were seen with mucin from the intestinal tract. A. salmonicida bound to the mucins isolated from the intestinal tract to a greater extent than to the skin mucins. The mucins from the intestinal regions had higher levels of sialylation than the skin mucins. Desialylating intestinal mucins decreased A. salmonicida binding, whereas desialylation of skin mucins resulted in complete loss of binding. In line with this, A. salmonicida also bound better to mammalian mucins with high levels of sialylation, and N-Acetylneuraminic acid appeared to be the sialic acid whose presence was imperative for binding. Thus, sialylated structures are important for A. salmonicida binding, suggesting a pivotal role for sialylation in mucosal defense. The marked differences in sialylation as well as A. salmonicida binding between the skin and intestinal tract suggest interorgan differences in the host-pathogen interaction and in the mucin defense against A. salmonicida.


