Sephin1Selective PPP1R15A inhibitor CAS# 13098-73-2 |
- Calcineurin Autoinhibitory Peptide
Catalog No.:BCC2456
CAS No.:148067-21-4
- BVT 948
Catalog No.:BCC2467
CAS No.:39674-97-0
- NSC 87877
Catalog No.:BCC2468
CAS No.:56990-57-9
- BX795
Catalog No.:BCC3635
CAS No.:702675-74-9
- Nafamostat
Catalog No.:BCC4187
CAS No.:81525-10-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13098-73-2 | SDF | Download SDF |
| PubChem ID | 248404 | Appearance | Powder |
| Formula | C8H9ClN4 | M.Wt | 196.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >7.75mg/mL in DMSO | ||
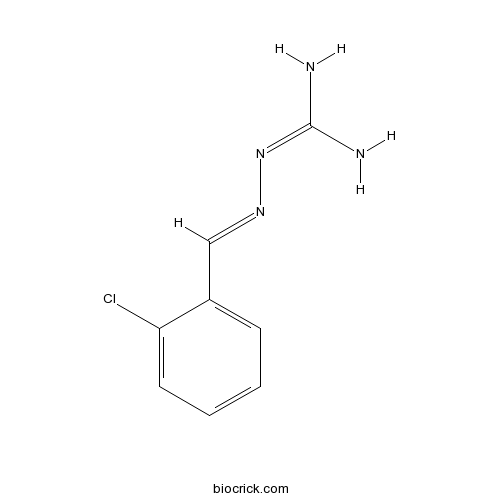
| Chemical Name | 2-[(2-chlorophenyl)methylideneamino]guanidine | ||
| SMILES | NC(N)=NN=Cc1ccccc1Cl | ||
| Standard InChIKey | PDWJALXSRRSUHR-LFYBBSHMSA-N | ||
| Standard InChI | InChI=1S/C8H9ClN4/c9-7-4-2-1-3-6(7)5-12-13-8(10)11/h1-5H,(H4,10,11,13)/b12-5+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Cell experiment [1]: | |
| Cell lines | HeLa and 293T cells |
| Preparation method | Limited solubility. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
| Reacting condition | 6 h |
| Applications | Sephin1 selectively disrupts the PPP1R15A-PP1c complex but leave the related PPP1R15B-PP1c complex unaffected. Thus Sephin1 prolongs eIF2a phosphorylation after stress, delays translation recover and attenuates expression of stress genes such as pro-apoptotic protein-CHOP. |
| Animal experiment [1]: | |
| Animal models | SOD1 mice SOD1G93A in C57BL/6J |
| Dosage form | 5 mg/kg |
| Application | The progressive weight loss of SOD1 mutant mice and their motor deficits are almost completely prevented by 5 mg/kg of Sephin1 once a day, without adverse effects on weight gain or motor performance of wild-type mice. Sephin1 also prevents the motor deficits of SOD1 mutant mice that associated with motor neuron loss. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: 1. Das I, Krzyzosiak A, Schneider K et al. Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science. 2015 Apr 10;348(6231):239-42. | |

Sephin1 Dilution Calculator

Sephin1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.0854 mL | 25.4272 mL | 50.8544 mL | 101.7087 mL | 127.1359 mL |
| 5 mM | 1.0171 mL | 5.0854 mL | 10.1709 mL | 20.3417 mL | 25.4272 mL |
| 10 mM | 0.5085 mL | 2.5427 mL | 5.0854 mL | 10.1709 mL | 12.7136 mL |
| 50 mM | 0.1017 mL | 0.5085 mL | 1.0171 mL | 2.0342 mL | 2.5427 mL |
| 100 mM | 0.0509 mL | 0.2543 mL | 0.5085 mL | 1.0171 mL | 1.2714 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sephin1 is a selective inhibitor of stress-induced PPP1R15A and targets disease associated with accumulation of misfolded protein. [1]
PPP1R15A is a regulator subunit of protein phosphatase 1 and regulates stress-induced eIF2α (α subunit of eukaryotic translation initiation factor 2). It brings PP1 (serine/threonine-protein phosphatase) to dephosphorylate eIF2α. Phosphorylation of eIF2α reduces protein synthesis and prevents the accumulation of misfolded protein in ER (endoplasmic reticulum). Thus inhibiting PPP1R15A prolongs the phosphorylation of eIF2α, and benefits the treatment for protein misfolding disease. [1]
In cells treated with 5μm Sephin1 for 6 hours, Sephin1 specifically disrupted the PPP1R15A-PP1c complex without affecting PPP115B-PP1c complex. As a consequence, Sephin1 prolonged eIF2a phosphorylation in HeLa cells after stress (*2.5 μg/ml tunicamycin treatment) and delayed translational recovery. Sephin1 recused cell from cytotoxic ER stress* in wild-type cell but not the PPP1R15A mutant cell. [1]
In mice administrated orally with 1-5mg/kg Sephin1 for given time, Sephin1 exhibited no adverse effects on rotarod performances, total body weight gain or memory. Treating MPZ (Deletion of serine 63 of myelin protein zero) mutant mice with 1mg/kg orally twice a day, prevented the molecular, morphological and motor defects. Oral treatment of 5 mg/kg of Sephin1 once a day, prevented motor deficits, motor neuron loss and the molecular defects in SOD1 (Mutant and misfolding-prone superoxide dismutase 1) mutant mice. [1]
Reference:
1: Das I, Krzyzosiak A, Schneider K, Wrabetz L, D'Antonio M, Barry N, Sigurdardottir A, Bertolotti A. Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science. 2015 Apr 10; 348(6231):239-42.
- (±)-CPSI 1306
Catalog No.:BCC6161
CAS No.:1309793-47-2
- H 89 2HCl
Catalog No.:BCC4997
CAS No.:130964-39-5
- Marumoside A
Catalog No.:BCN7702
CAS No.:1309604-34-9
- Wittifuran X
Catalog No.:BCN4794
CAS No.:1309478-07-6
- K145
Catalog No.:BCC4305
CAS No.:1309444-75-4
- GPR40 Activator 1
Catalog No.:BCC4125
CAS No.:1309435-60-6
- Cerberic acid B
Catalog No.:BCN4715
CAS No.:1309362-77-3
- CX-4945 sodium salt
Catalog No.:BCC5586
CAS No.:1309357-15-0
- Entacapone
Catalog No.:BCC2217
CAS No.:130929-57-6
- 7-O-Acetylneocaesalpin N
Catalog No.:BCN7332
CAS No.:1309079-08-0
- N4-Benzoylcytidine
Catalog No.:BCC9072
CAS No.:13089-48-0
- Fmoc-Val-OSu
Catalog No.:BCC3572
CAS No.:130878-68-1
- MRS 4062 triethylammonium salt
Catalog No.:BCC6134
CAS No.:1309871-50-8
- 15-Methoxymkapwanin
Catalog No.:BCN6498
CAS No.:1309920-99-7
- VU 0360172 hydrochloride
Catalog No.:BCC6141
CAS No.:1309976-62-2
- alpha-Yohimbine
Catalog No.:BCN6166
CAS No.:131-03-3
- Dimethyl phthalate
Catalog No.:BCN6167
CAS No.:131-11-3
- Pimpinellin
Catalog No.:BCN6168
CAS No.:131-12-4
- N-Acetylneuraminic acid
Catalog No.:BCN2204
CAS No.:131-48-6
- Oxybenzone
Catalog No.:BCC5445
CAS No.:131-57-7
- Meptyldinocap
Catalog No.:BCC5468
CAS No.:131-72-6
- 8-Epiloganic acid
Catalog No.:BCC8956
CAS No.:82509-41-9
- Boldenone undecylenate
Catalog No.:BCC8896
CAS No.:13103-34-9
- GNF179 Metabolite
Catalog No.:BCC5176
CAS No.:1310455-86-7
Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit.[Pubmed:25859045]
Science. 2015 Apr 10;348(6231):239-42.
Protein phosphorylation regulates virtually all biological processes. Although protein kinases are popular drug targets, targeting protein phosphatases remains a challenge. Here, we describe Sephin1 (selective inhibitor of a holophosphatase), a small molecule that safely and selectively inhibited a regulatory subunit of protein phosphatase 1 in vivo. Sephin1 selectively bound and inhibited the stress-induced PPP1R15A, but not the related and constitutive PPP1R15B, to prolong the benefit of an adaptive phospho-signaling pathway, protecting cells from otherwise lethal protein misfolding stress. In vivo, Sephin1 safely prevented the motor, morphological, and molecular defects of two otherwise unrelated protein-misfolding diseases in mice, Charcot-Marie-Tooth 1B, and amyotrophic lateral sclerosis. Thus, regulatory subunits of phosphatases are drug targets, a property exploited here to safely prevent two protein misfolding diseases.
PPP1R15A-mediated dephosphorylation of eIF2alpha is unaffected by Sephin1 or Guanabenz.[Pubmed:28447936]
Elife. 2017 Apr 27;6.
Dephosphorylation of translation initiation factor 2 (eIF2alpha) terminates signalling in the mammalian integrated stress response (ISR) and has emerged as a promising target for modifying the course of protein misfolding diseases. The [(o-chlorobenzylidene)amino]guanidines (Guanabenz and Sephin1) have been proposed to exert protective effects against misfolding by interfering with eIF2alpha-P dephosphorylation through selective disruption of a PP1-PPP1R15A holophosphatase complex. Surprisingly, they proved inert in vitro affecting neither stability of the PP1-PPP1R15A complex nor substrate-specific dephosphorylation. Furthermore, eIF2alpha-P dephosphorylation, assessed by a kinase shut-off experiment, progressed normally in Sephin1-treated cells. Consistent with its role in defending proteostasis, Sephin1 attenuated the IRE1 branch of the endoplasmic reticulum unfolded protein response. However, repression was noted in both wildtype and Ppp1r15a deleted cells and in cells rendered ISR-deficient by CRISPR editing of the Eif2s1 locus to encode a non-phosphorylatable eIF2alpha (eIF2alpha(S51A)). These findings challenge the view that [(o-chlorobenzylidene)amino]guanidines restore proteostasis by interfering with eIF2alpha-P dephosphorylation.
Decoding the selectivity of eIF2alpha holophosphatases and PPP1R15A inhibitors.[Pubmed:28759048]
Nat Struct Mol Biol. 2017 Sep;24(9):708-716.
The reversible phosphorylation of proteins controls most cellular functions. Protein kinases have been popular drug targets, unlike phosphatases, which remain a drug discovery challenge. Guanabenz and Sephin1 are selective inhibitors of the phosphatase regulatory subunit PPP1R15A (R15A) that prolong the benefit of eIF2alpha phosphorylation, thereby protecting cells from proteostatic defects. In mice, Sephin1 prevents two neurodegenerative diseases, Charcot-Marie-Tooth 1B (CMT-1B) and SOD1-mediated amyotrophic lateral sclerosis (ALS). However, the molecular basis for R15A inhibition is unknown. Here we reconstituted human recombinant eIF2alpha holophosphatases, R15A-PP1 and R15B-PP1, whose activity depends on both the catalytic subunit PP1 (protein phosphatase 1) and either R15A or R15B. This system enabled the functional characterization of these holophosphatases and revealed that Guanabenz and Sephin1 induced a selective conformational change in R15A, detected by resistance to limited proteolysis. This altered the recruitment of eIF2alpha, preventing its dephosphorylation. This work demonstrates that regulatory subunits of phosphatases are valid drug targets and provides the molecular rationale to expand this concept to other phosphatases.


