CX-5461Pol I-mediated rRNA synthesis inhibitor CAS# 1138549-36-6 |
- Cytarabine
Catalog No.:BCC3759
CAS No.:147-94-4
- Mithramycin A
Catalog No.:BCC2470
CAS No.:18378-89-7
- Mercaptopurine (6-MP)
Catalog No.:BCC1186
CAS No.:50-44-2
- Rifaximin (Xifaxan)
Catalog No.:BCC3848
CAS No.:80621-81-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1138549-36-6 | SDF | Download SDF |
| PubChem ID | 25257557 | Appearance | Powder |
| Formula | C27H27N7O2S | M.Wt | 513.61 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : < 1 mg/mL (insoluble or slightly soluble) | ||
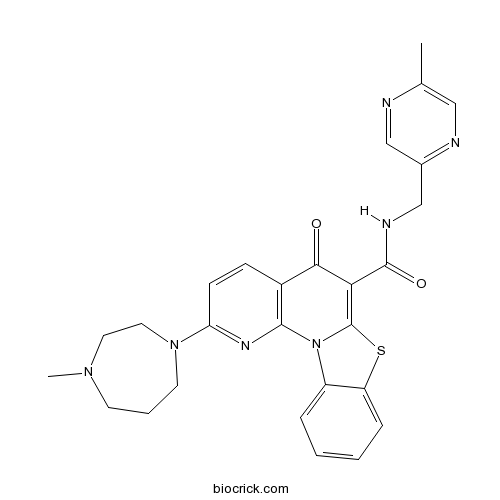
| Chemical Name | 2-(4-methyl-1,4-diazepan-1-yl)-N-[(5-methylpyrazin-2-yl)methyl]-5-oxo-[1,3]benzothiazolo[3,2-a][1,8]naphthyridine-6-carboxamide | ||
| SMILES | CC1=CN=C(C=N1)CNC(=O)C2=C3N(C4=CC=CC=C4S3)C5=C(C2=O)C=CC(=N5)N6CCCN(CC6)C | ||
| Standard InChIKey | XGPBJCHFROADCK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H27N7O2S/c1-17-14-29-18(15-28-17)16-30-26(36)23-24(35)19-8-9-22(33-11-5-10-32(2)12-13-33)31-25(19)34-20-6-3-4-7-21(20)37-27(23)34/h3-4,6-9,14-15H,5,10-13,16H2,1-2H3,(H,30,36) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | CX-5461 is an inhibitor of rRNA synthesis with IC50 of 142 nM for Pol I-driven transcription of rRNA, | ||||||
| Targets | Pol I | HCT-116 | A375 | MIA PaCa-2 | |||
| IC50 | 142 nM | 167 nM (ED50) | 58 nM (ED50) | 74 nM (ED50) | |||

CX-5461 Dilution Calculator

CX-5461 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.947 mL | 9.735 mL | 19.47 mL | 38.9401 mL | 48.6751 mL |
| 5 mM | 0.3894 mL | 1.947 mL | 3.894 mL | 7.788 mL | 9.735 mL |
| 10 mM | 0.1947 mL | 0.9735 mL | 1.947 mL | 3.894 mL | 4.8675 mL |
| 50 mM | 0.0389 mL | 0.1947 mL | 0.3894 mL | 0.7788 mL | 0.9735 mL |
| 100 mM | 0.0195 mL | 0.0974 mL | 0.1947 mL | 0.3894 mL | 0.4868 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CX-5461 is a potent and orally bioavailable small-molecule inhibitor of rRNA synthesis that specifically inhibits RNA polymerase (Pol) I-driven transcription with IC50 value of 142 nM. CX-5461 exhibits antiproliferative activity against human pancreatic tumor cells MIA Paca-2, human melanoma cells A375 and colorectal carcinoma cells HCT-116 with EC50 values of 74, 58, and 167 nmol/L, respectively. [1].
CX-5461 was revealed to inhibit Pol I transcription via promoting the stabilization of p53. In addition, CX-5461 has been demonstrated to induce autophagy and senescence but not apoptosis in MIA Paca-2 and A375 cell lines.
In vivo, CX-5461 has shown to suppress tumor volume in both MIA Paca-2 and A375 derived xenograft mice models [1].
References:
[1] Drygin D1, Lin A, Bliesath J, Ho CB, O'Brien SE, Proffitt C, Omori M, Haddach M, Schwaebe MK, Siddiqui-Jain A, Streiner N, Quin JE, Sanij E, Bywater MJ,Hannan RD, Ryckman D, Anderes K, Rice WG. Targeting RNA polymerase I with an oral small molecule CX-5461 inhibits ribosomal RNA synthesis and solid tumor growth. Cancer Res. 2011 Feb 15;71(4):1418-30
- Cidofovir
Catalog No.:BCC2546
CAS No.:113852-37-2
- N-p-coumaroyl-N'-caffeoylputrescine
Catalog No.:BCN6018
CAS No.:1138156-77-0
- N1,N12-Diethylspermine tetrahydrochloride
Catalog No.:BCC6669
CAS No.:113812-15-0
- Picrasidine T
Catalog No.:BCN6017
CAS No.:113808-03-0
- Z-Gly-OH
Catalog No.:BCC2770
CAS No.:1138-80-3
- (Z)-FeCP-oxindole
Catalog No.:BCC6079
CAS No.:1137967-28-2
- TAK960
Catalog No.:BCC6411
CAS No.:1137868-52-0
- MDL 72832 hydrochloride
Catalog No.:BCC6637
CAS No.:113777-40-5
- Dexmedetomidine
Catalog No.:BCC4326
CAS No.:113775-47-6
- Eudesm-4(15)-ene-3alpha,11-diol
Catalog No.:BCN4060
CAS No.:113773-90-3
- Ilexhainanoside D
Catalog No.:BCN7863
CAS No.:1137648-52-2
- LX1606
Catalog No.:BCC1713
CAS No.:1137608-69-5
- Cinnamyl 3-aminobut-2-enoate
Catalog No.:BCC8914
CAS No.:113898-97-8
- Caryophyllene oxide
Catalog No.:BCN6019
CAS No.:1139-30-6
- Koumine N-oxide
Catalog No.:BCN4807
CAS No.:113900-75-7
- PNU 74654
Catalog No.:BCC7704
CAS No.:113906-27-7
- PMPA (NMDA antagonist)
Catalog No.:BCC7308
CAS No.:113919-36-1
- Andrographidine C
Catalog No.:BCN4730
CAS No.:113963-39-6
- Andrographidine E
Catalog No.:BCN4729
CAS No.:113963-41-0
- (Z)-Akuammidine
Catalog No.:BCN6020
CAS No.:113973-31-2
- Cefozopran hydrochloride
Catalog No.:BCC8909
CAS No.:113981-44-5
- 5-Hydroxy-7,8-dimethoxyflavanone
Catalog No.:BCN6021
CAS No.:113981-49-0
- Golgicide A
Catalog No.:BCC4373
CAS No.:1139889-93-2
- Erythromycin
Catalog No.:BCC4778
CAS No.:114-07-8
RNA Polymerase I Inhibition with CX-5461 as a Novel Therapeutic Strategy to Target MYC in Multiple Myeloma.[Pubmed:28369725]
Br J Haematol. 2017 Apr;177(1):80-94.
Dysregulation of MYC is frequently implicated in both early and late myeloma progression events, yet its therapeutic targeting has remained a challenge. Among key MYC downstream targets is ribosomal biogenesis, enabling increases in protein translational capacity necessary to support the growth and self-renewal programmes of malignant cells. We therefore explored the selective targeting of ribosomal biogenesis with the small molecule RNA polymerase (pol) I inhibitor CX-5461 in myeloma. CX-5461 induced significant growth inhibition in wild-type (WT) and mutant TP53 myeloma cell lines and primary samples, in association with increases in downstream markers of apoptosis. Moreover, Pol I inhibition overcame adhesion-mediated drug resistance and resistance to conventional and novel agents. To probe the TP53-independent mechanisms of CX-5461, gene expression profiling was performed on isogenic TP53 WT and knockout cell lines and revealed reduction of MYC downstream targets. Mechanistic studies confirmed that CX-5461 rapidly suppressed both MYC protein and MYC mRNA levels. The latter was associated with an increased binding of the RNA-induced silencing complex (RISC) subunits TARBP2 and AGO2, the ribosomal protein RPL5, and MYC mRNA, resulting in increased MYC transcript degradation. Collectively, these studies provide a rationale for the clinical translation of CX-5461 as a novel therapeutic approach to target MYC in myeloma.
CX-5461 is a DNA G-quadruplex stabilizer with selective lethality in BRCA1/2 deficient tumours.[Pubmed:28211448]
Nat Commun. 2017 Feb 17;8:14432.
G-quadruplex DNAs form four-stranded helical structures and are proposed to play key roles in different cellular processes. Targeting G-quadruplex DNAs for cancer treatment is a very promising prospect. Here, we show that CX-5461 is a G-quadruplex stabilizer, with specific toxicity against BRCA deficiencies in cancer cells and polyclonal patient-derived xenograft models, including tumours resistant to PARP inhibition. Exposure to CX-5461, and its related drug CX-3543, blocks replication forks and induces ssDNA gaps or breaks. The BRCA and NHEJ pathways are required for the repair of CX-5461 and CX-3543-induced DNA damage and failure to do so leads to lethality. These data strengthen the concept of G4 targeting as a therapeutic approach, specifically for targeting HR and NHEJ deficient cancers and other tumours deficient for DNA damage repair. CX-5461 is now in advanced phase I clinical trial for patients with BRCA1/2 deficient tumours (Canadian trial, NCT02719977, opened May 2016).
Therapeutic Targeting of RNA Polymerase I With the Small-Molecule CX-5461 for Prevention of Arterial Injury-Induced Neointimal Hyperplasia.[Pubmed:28062495]
Arterioscler Thromb Vasc Biol. 2017 Mar;37(3):476-484.
OBJECTIVE: RNA polymerase I (Pol I)-dependent rRNA synthesis is a determinant factor in ribosome biogenesis and thus cell proliferation. The importance of dysregulated Pol I activity in cardiovascular disease, however, has not been recognized. Here, we tested the hypothesis that specific inhibition of Pol I might prevent arterial injury-induced neointimal hyperplasia. APPROACH AND RESULTS: CX-5461 is a novel selective Pol I inhibitor. Using this tool, we demonstrated that local inhibition of Pol I blocked balloon injury-induced neointima formation in rat carotid arteries in vivo. Neointimal development was associated with augmented rDNA transcriptional activity as evidenced by the increased phosphorylation of upstream binding factor-1. The beneficial effect of CX-5461 was mainly mediated by inducing G2/M cell cycle arrest of proliferating smooth muscle cells without obvious apoptosis. CX-5461 did not induce p53 stabilization but increased p53 phosphorylation and acetylation and activated the ataxia telangiectasia mutated/ataxia telangiectasia and Rad3-related (ATR) pathway. Inhibition of ATR, but not of ataxia telangiectasia mutated, abolished the cytostatic effect of CX-5461 and p53 phosphorylation. In addition, inhibition of p53 or knockdown of the p53 target GADD45 mimicked the effect of ATR inhibition. In vivo experiments showed that the levels of phospho-p53 and acetyl-p53, and activity of the ataxia telangiectasia mutated/ATR pathway were all augmented in CX-5461-treated vessels. CONCLUSIONS: Pol I can be therapeutically targeted to inhibit the growth of neointima, supporting that Pol I is a novel biological target for preventing arterial restenosis. Mechanistically, Pol I inhibition elicited G2/M cell cycle arrest in smooth muscle cells via activation of the ATR-p53 axis.
CX-5461 induces autophagy and inhibits tumor growth via mammalian target of rapamycin-related signaling pathways in osteosarcoma.[Pubmed:27729807]
Onco Targets Ther. 2016 Sep 29;9:5985-5997.
Osteosarcoma (OS) is the most common primary bone tumor, but molecular mechanisms of the disease have not been well understood, and treatment of metastatic OS remains a challenge. Rapid ribosomal RNA synthesis in cancer is transcribed by RNA polymerase I, which results in unbridled cell growth. The recent discovery of CX-5461, a selective RNA polymerase I inhibitor, exerted its inhibitory effect of ribosomal RNA synthesis and antiproliferative potency. Here, we demonstrate that CX-5461 induces G2 arrest in the cell cycle and expression of microtubule-associated protein 1 light chain 3 II isoform in OS cell lines. Autophagic vacuoles could be observed in electron microscopy and 3-methyladenine prevented cell death mediated by CX-5461. Moreover, it significantly augmented phosphorylated AMP-Activated Protein Kinases alpha (p-AMPK alpha). (Thr(172)) expression in U2-OS cells and decreased p-Akt (Ser(473)) expression in MNNG cells, respectively, which repressed their downstream effector, mammalian target of rapamycin. On the other hand, CX-5461 increased p53 accumulation and messenger RNA level of its target genes, p21, MDM2, and Sestrin1/2 in U2-OS cells. Knockdown of p53 expression markedly impaired cell death as well as the expression of light chain 3-II and p21 induced by CX-5461. It also significantly enhanced doxorubicin-mediated cytotoxic effect in vitro and in vivo together with additive expression of p53, p21, and light chain 3-II in U2-OS cells. Our data indicate that CX-5461 might induce autophagy via mammalian target of rapamycin-associated signaling pathways dependent on p53 status and exert p53-dependent synergistic antitumor effect combined with doxorubicin in OS. These results suggest that CX-5461 might be promising in clinical therapy for OS, especially cases harboring wild-type p53.


