CidofovirAnti-CMV drug;inhibitor of viral DNA syntheis CAS# 113852-37-2 |
- Adefovir Dipivoxil
Catalog No.:BCC5025
CAS No.:142340-99-6
- Valganciclovir HCl
Catalog No.:BCC4745
CAS No.:175865-59-5
- CMX001
Catalog No.:BCC4106
CAS No.:444805-28-1
- Valganciclovir
Catalog No.:BCC2026
CAS No.:88110-89-8
- Letermovir
Catalog No.:BCC1700
CAS No.:917389-32-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 113852-37-2 | SDF | Download SDF |
| PubChem ID | 466146 | Appearance | Powder |
| Formula | C8H14N3O6P | M.Wt | 279.19 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GS 0504; HPMPC; (S)-HPMPC | ||
| Solubility | Soluble to 12 mg/mL (42.98 mM) in Water | ||
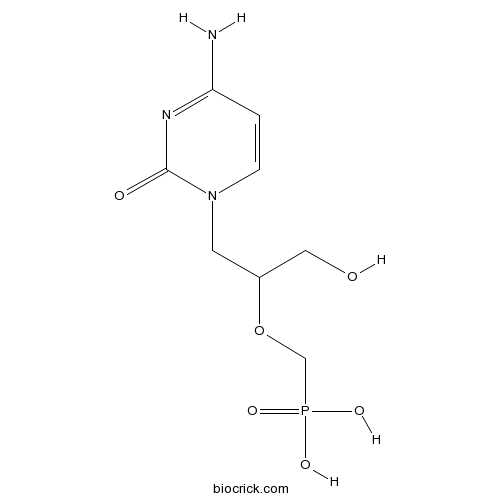
| Chemical Name | [1-(4-amino-2-oxopyrimidin-1-yl)-3-hydroxypropan-2-yl]oxymethylphosphonic acid | ||
| SMILES | C1=CN(C(=O)N=C1N)CC(CO)OCP(=O)(O)O | ||
| Standard InChIKey | VWFCHDSQECPREK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H14N3O6P/c9-7-1-2-11(8(13)10-7)3-6(4-12)17-5-18(14,15)16/h1-2,6,12H,3-5H2,(H2,9,10,13)(H2,14,15,16) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cidofovir is an anti-CMV drug which can suppress CMV replication by selective inhibition of viral DNA polymerase and therefore prevention of viral replication and transcription.
IC50 Value:
Target: CMV DNA polymerase
in vitro: The minimum concentrations of (S)-HPMPC required to inhibit CMV plaque formation by 50% was microgram/ml. The selectivity indices of (S)-HPMPC, as determined by the ratio of the 50% inhibitory concentration for cell growth to the 50% inhibitory concentration for plaque formation for CMV (AD-169 strain), was 1,500 [1]. The time course of uptake of HPMPC into Vero cells was linear between 10 and 75 min and proportional to the concentration in the medium from 10(-6) to 10(-2) M. HPMPC uptake was temperature sensitive and the rate of uptake was considerably lower at 27 degrees than at 37 degrees and almost totally inhibited at 4 degrees [2].
in vivo: Levels of cidofovirin serum following intravenous infusion were dose proportional over the dose range of 1.0 to 10.0 mg/kg of body weight and declined biexponentially with an overall mean +/- standard deviation terminal half-life of 2.6 +/- 1.2 h (n = 25). Approximately 90% of the intravenous dose was recovered unchanged in the urine in 24 h. The overall mean +/- standard deviation total clearance of the drug from serum (148 +/- 25 ml/h/kg; n = 25) approximated renal clearance (129 +/- 42 ml/h/kg; n = 25), which was significantly higher (P < 0.001) than the baseline creatinine clearance in the same patients (83 +/- 21 ml/h/kg; n = 12) [3]. Positive CMV urine cultures reverted to negative in 2 of 8 patients receiving doses of < or = 1.5 mg/kg twice weekly and 11 of 13 patients receiving higher doses. Cidofovir has in vivo anti-CMV activity demonstrated by prolonged clearing of CMV viruria, although this observation is tempered by the fact that clearance of viremia could not be demonstrated [4].
Toxicity: Patients receiving 0.5 or 1.5 mg/kg twice weekly experienced no serious toxicity. The first two patients who received 5 mg/kg twice weekly developed glycosuria and 2+ proteinuria. Subsequent patients received concomitant probenecid to attempt to ameliorate renal toxicity [4].
Clinical trial: FDA approved drug References: | |||||

Cidofovir Dilution Calculator

Cidofovir Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5818 mL | 17.909 mL | 35.8179 mL | 71.6358 mL | 89.5448 mL |
| 5 mM | 0.7164 mL | 3.5818 mL | 7.1636 mL | 14.3272 mL | 17.909 mL |
| 10 mM | 0.3582 mL | 1.7909 mL | 3.5818 mL | 7.1636 mL | 8.9545 mL |
| 50 mM | 0.0716 mL | 0.3582 mL | 0.7164 mL | 1.4327 mL | 1.7909 mL |
| 100 mM | 0.0358 mL | 0.1791 mL | 0.3582 mL | 0.7164 mL | 0.8954 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cidofovir(Vistide) is an injectable antiviral medication for the treatment of cytomegalovirus (CMV) retinitis. (IC50= 0.94 μM) A wild type HCMV laboratory strain (AD 169) and 3 ganciclovir-resistant clinical isolates(generated as a result of Ganciclovir t
- N-p-coumaroyl-N'-caffeoylputrescine
Catalog No.:BCN6018
CAS No.:1138156-77-0
- N1,N12-Diethylspermine tetrahydrochloride
Catalog No.:BCC6669
CAS No.:113812-15-0
- Picrasidine T
Catalog No.:BCN6017
CAS No.:113808-03-0
- Z-Gly-OH
Catalog No.:BCC2770
CAS No.:1138-80-3
- (Z)-FeCP-oxindole
Catalog No.:BCC6079
CAS No.:1137967-28-2
- TAK960
Catalog No.:BCC6411
CAS No.:1137868-52-0
- MDL 72832 hydrochloride
Catalog No.:BCC6637
CAS No.:113777-40-5
- Dexmedetomidine
Catalog No.:BCC4326
CAS No.:113775-47-6
- Eudesm-4(15)-ene-3alpha,11-diol
Catalog No.:BCN4060
CAS No.:113773-90-3
- Ilexhainanoside D
Catalog No.:BCN7863
CAS No.:1137648-52-2
- LX1606
Catalog No.:BCC1713
CAS No.:1137608-69-5
- BRD 7552
Catalog No.:BCC8035
CAS No.:1137359-47-7
- CX-5461
Catalog No.:BCC3700
CAS No.:1138549-36-6
- Cinnamyl 3-aminobut-2-enoate
Catalog No.:BCC8914
CAS No.:113898-97-8
- Caryophyllene oxide
Catalog No.:BCN6019
CAS No.:1139-30-6
- Koumine N-oxide
Catalog No.:BCN4807
CAS No.:113900-75-7
- PNU 74654
Catalog No.:BCC7704
CAS No.:113906-27-7
- PMPA (NMDA antagonist)
Catalog No.:BCC7308
CAS No.:113919-36-1
- Andrographidine C
Catalog No.:BCN4730
CAS No.:113963-39-6
- Andrographidine E
Catalog No.:BCN4729
CAS No.:113963-41-0
- (Z)-Akuammidine
Catalog No.:BCN6020
CAS No.:113973-31-2
- Cefozopran hydrochloride
Catalog No.:BCC8909
CAS No.:113981-44-5
- 5-Hydroxy-7,8-dimethoxyflavanone
Catalog No.:BCN6021
CAS No.:113981-49-0
- Golgicide A
Catalog No.:BCC4373
CAS No.:1139889-93-2
Reduced cell viability and apoptosis induction in human thyroid carcinoma and mesothelioma cells exposed to cidofovir.[Pubmed:28223140]
Toxicol In Vitro. 2017 Jun;41:49-55.
Besides its well-recognized antiviral activity, Cidofovir (CDV) has been shown to exert anticancer properties both within in vitro and in vivo models. The aim of this study was to evaluate the effects of CDV on still unexplored cultured cancer cells from human mesothelioma as well as breast, colon, liver, lung, prostate, and thyroid carcinomas. Overall, a dose- and time-dependent inhibition of cell viability was observed after CDV exposure. To clarify the mechanisms underlying CDV action, apoptotic cell death was investigated in two infected cell lines [Ist-Mes1 and Ist-Mes2 mesothelioma cells (SV40+)] and in two uninfected cell lines (NCI-H2425 mesothelioma cells and FTC-133 thyroid cancer cells), which resulted the most sensitive to CDV treatment. Reduced expression of procaspase-3 and increased expression of PARP p85 fragment were observed in both infected and uninfected mesothelioma cells, indicating apoptosis induction by CDV in a virus-independent manner. Similarly, the increase of the pro-apoptotic proteins p53, cytochrome c and caspase-3, the decrease of the survival protein Bcl-x, and the increment of Bax/Bcl-2 ratio revealed the occurrence of apoptosis in CDV-treated FTC-133. The presence of nuclear DNA fragmentation confirmed apoptotic cell death by CDV. Overall, our findings warrant further investigations to explore the therapeutic potential of CDV for human mesothelioma and follicular thyroid carcinoma.
Comparison of cidofovir and the measles, mumps, and rubella vaccine in the treatment of recurrent respiratory papillomatosis.[Pubmed:28231366]
Ear Nose Throat J. 2017 Feb;96(2):69-74.
We conducted a retrospective study of the use of Cidofovir and the measles, mumps, and rubella (MMR) vaccineas adjunctive treatments to lesion debridement in patients with recurrent respiratory papillomatosis (RRP). Our study population was made up of 15 children-7 boys and 8 girls, aged 1 to 16 years at diagnosis (mean: 6.2)-with pathologically confirmed RRP who had been followed for at least 1 year. In addition to demographic data, we compiled information on disease severity, the type of adjunctive treatment administered to each patient, the frequency of debridements, the length of observation, and remission rates. Of the 15 patients, 5 had been treated with Cidofovirafter debridement (Cidofovir-only group), 6 were treated with MMR vaccine after debridement (MMR-only group), 3 were treated with one and later switched to the other based on parental preference, and 1 received neither treatment, only debridement. The initial mean Derkay disease severity scores were 12.6 for the Cidofovir-only group and 11.0 for the MMR-only group (p = 0.61). The Cidofovir-only patients underwent an average of 11.8 adjunctive treatments and the MMR-only patients an average of 17.7 (p = 0.33). The average duration of observation was 44.0 months in the Cidofovir-only group and 64.7 months in the MMR-only group (p = 0.29). Remission rates were 20% in the Cidofovir-only group and 50% in the MMR-only group (p = 0.54). Our study found insufficient evidence of any significant differences between Cidofovir and the MMR vaccinein terms of the number and frequency of adjunctive treatments and the rates of remission.


