CMX001Broad spectrum antiviral drug CAS# 444805-28-1 |
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- FMK
Catalog No.:BCC1580
CAS No.:821794-92-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

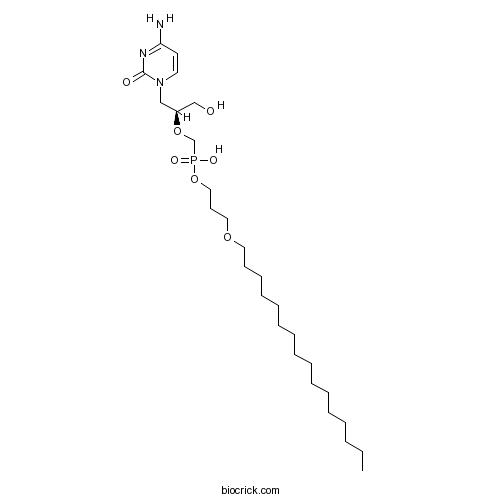
| Cas No. | 444805-28-1 | SDF | Download SDF |
| PubChem ID | 483477 | Appearance | Powder |
| Formula | C27H52N3O7P | M.Wt | 561.69 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Brincidofovir; HDP-cidofovir; Hexadecyloxypropyl-cidofovir | ||
| Solubility | Soluble in DMSO | ||
| Chemical Name | [(2S)-1-(4-amino-2-oxopyrimidin-1-yl)-3-hydroxypropan-2-yl]oxymethyl-(3-hexadecoxypropoxy)phosphinic acid | ||
| SMILES | CCCCCCCCCCCCCCCCOCCCOP(=O)(COC(CN1C=CC(=NC1=O)N)CO)O | ||
| Standard InChIKey | WXJFKKQWPMNTIM-VWLOTQADSA-N | ||
| Standard InChI | InChI=1S/C27H52N3O7P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-19-35-20-16-21-37-38(33,34)24-36-25(23-31)22-30-18-17-26(28)29-27(30)32/h17-18,25,31H,2-16,19-24H2,1H3,(H,33,34)(H2,28,29,32)/t25-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | CMX001 (Brincidofovir; HDP-CDV) was developed as an orally active, lipophilic form of cidofovir (CDV); has enhanced activity in vitro and in vivo compared to CDV against certain herpesviruses, adenoviruses and orthopoxviruses.
IC50 Value: 5.5 nM (EC50, in PDA at 7 dpi) [3]
Target: anti-CMV
CMX001 is currently in Phase II clinical studies for development as a therapeutic agent for human CMV, adenovirus and BK virus infections, as well as, for adverse events following smallpox vaccinations.
in vitro: In PDA at 7 dpi, the CMX001 50% effective concentration (EC50) was 5.55 nM, the 50% cytotoxic concentration (CC50) was 184.6 nM, and the 50% selectivity index (SI50) was 33.3. The EC90 was 19.7 nM, the CC90 was 5,054 nM, and the SI90 was 256.1. In COS-7 cells, JCV replication was faster and the EC50 and EC90 were 18- and 37-fold higher than those in PDA, i.e., 0.1 μM and 0.74 μM (CC50, 0.67 μM; SI50, 6.7; CC90, 12.2 μM; SI90, 16.5) at 5 dpi [3].
in vivo: CMX001 and CDV are equally efficacious at protecting mice from mortality following high ectromelia virus doses (10,000 x LD(50)) introduced by the intra-nasal route or small particle aerosol. Using CMX001 at a 10mg/kg dose followed by 2.5mg/kg doses every other-day for 14 days provided solid protection against mortality and weight loss following an intra-nasal challenge of (100-200) x LD(50) of ectromelia virus [1]. When CMX001 was administered orally to mice infected with HSV-1, mortality was reduced significantly (p≤0.001) with all three dose levels when treatments were initiated 24 h post viral inoculation. When treatments were started 48 h post viral inoculation, 5 and 2.5 mg/kg significantly reduced mortality (p≤ 0.001). If treatments were delayed until 72 h post viral inoculation, CMX001 did not reduce mortality or increase the mean day to death. When mice were infected intranasally with HSV-1 and treatments initiated 24 h post viral inoculation using CMX001 at 5 mg/kg or ACV at 100 mg/kg, virus replication in target organs was reduced by both CMX001 and ACV when compared to vehicle treated mice [2].
Toxicity: Diarrhea was the most common adverse event in patients receiving CMX001 at doses of 200 mg weekly or higher and was dose-limiting at 200 mg twice weekly. Myelosuppression and nephrotoxicity were not observed [4]. References: | |||||

CMX001 Dilution Calculator

CMX001 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7803 mL | 8.9017 mL | 17.8034 mL | 35.6068 mL | 44.5085 mL |
| 5 mM | 0.3561 mL | 1.7803 mL | 3.5607 mL | 7.1214 mL | 8.9017 mL |
| 10 mM | 0.178 mL | 0.8902 mL | 1.7803 mL | 3.5607 mL | 4.4509 mL |
| 50 mM | 0.0356 mL | 0.178 mL | 0.3561 mL | 0.7121 mL | 0.8902 mL |
| 100 mM | 0.0178 mL | 0.089 mL | 0.178 mL | 0.3561 mL | 0.4451 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CMX001 is a broad spectrum antiviral agent [1].
CMX001 has been reported to be active in vitro against a broad range of viruses from all five families of dsDNA viruses infecting human with EC50 values of 0.02μM, 0.0004μM, 17μM , 0.045μM and 0.07μM for Adenovirus, Herpesvirus, Papillomavirus, Polyomavirus and Orthopoxvirus, respectively. In addition, CMX001 has been revealed to arrest disease progression by inhibiting viral DNA replication and thereby reducing viral burden. Apart from these, initial studies has been demonstrated the efficacy of CMX001 for pre-exposure and post-exposure prophylaxis in mouse, rabbit, cynomolgus monkeys and human [1].
References:
[1]Lanier R1, Trost L, Tippin T, Lampert B, Robertson A, Foster S, Rose M, Painter W, O'Mahony R, Almond M, Painter G.Development of CMX001 for the Treatment of Poxvirus Infections. Viruses. 2010 Dec; 2(12):2740-2762.
- Pazopanib (GW-786034)
Catalog No.:BCC1286
CAS No.:444731-52-6
- Norwogonin
Catalog No.:BCN3190
CAS No.:4443-09-8
- 2-Cyclohexylethylamine
Catalog No.:BCN1794
CAS No.:4442-85-7
- 1,4-Benzodioxane-6-carboxylic acid
Catalog No.:BCC8422
CAS No.:4442-54-0
- Vandetanib (ZD6474)
Catalog No.:BCC3883
CAS No.:443913-73-3
- Glycosmisic acid
Catalog No.:BCN3650
CAS No.:443908-19-8
- Norkhellol
Catalog No.:BCN5497
CAS No.:4439-68-3
- 3-Chloro-4-(3-fluorobenzyloxy)nitrobenzene
Catalog No.:BCC8626
CAS No.:443882-99-3
- JNJ-7706621
Catalog No.:BCC2171
CAS No.:443797-96-4
- BC 11 hydrobromide
Catalog No.:BCC2381
CAS No.:443776-49-6
- Z-DL-Met-OH
Catalog No.:BCC2759
CAS No.:4434-61-1
- SF 11
Catalog No.:BCC7805
CAS No.:443292-81-7
- Cyclopamine
Catalog No.:BCN2964
CAS No.:4449-51-8
- Warangalone
Catalog No.:BCN4788
CAS No.:4449-55-2
- AM1241
Catalog No.:BCC2551
CAS No.:444912-48-5
- TC-O 9311
Catalog No.:BCC7900
CAS No.:444932-31-4
- EBPC
Catalog No.:BCC6677
CAS No.:4450-98-0
- 1,4:3,6-Dianhydro-α-D-glucopyranose
Catalog No.:BCC8420
CAS No.:4451-30-3
- ZM 449829
Catalog No.:BCC2444
CAS No.:4452-06-6
- BMS-345541(free base)
Catalog No.:BCC5374
CAS No.:445430-58-0
- SGS 518 oxalate
Catalog No.:BCC7750
CAS No.:445441-27-0
- BMS CCR2 22
Catalog No.:BCC7572
CAS No.:445479-97-0
- Homoeriodictyol
Catalog No.:BCN6804
CAS No.:446-71-9
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
Treatment of BK virus-associated nephropathy with CMX001 after kidney transplantation in a young child.[Pubmed:25174393]
Pediatr Transplant. 2014 Nov;18(7):E227-31.
NC, with renal failure secondary to bilateral dysplastic kidneys, received an LRD renal transplant (tx) at 17 months of age. Her early post-tx course was complicated by persistently elevated blood polyoma BK virus DNA loads. A protocol biopsy at six months post-transplant revealed BKVAN. Blood viral loads did not respond to decreased immunosuppression or treatment with ciprofloxacin and leflunomide. Six months post-tx, her serum creatinine began to rise and we sought experimental therapy to prevent the loss of her graft. At seven months post-tx, with FDA approval under an eIND, the patient was started on a 36-wk course of treatment with the investigational drug. The patient is now more than 24 months after stopping treatment with CMX. BKV viral DNA loads remain at low, but still detectable levels. Urine viral loads have declined, but remain elevated. EBV DNA loads become undetectable. The patient's serum creatinine has declined back to a baseline of 0.5-0.7 mg/dL and has been stable for two yr. Renal function was preserved in association with the use of CMX001 to treat BKV nephropathy in a young pediatric kidney transplant recipient. There were no serious adverse events associated with the use of CMX001. This novel medication may be of value in the treatment of BKVAN in pediatric renal transplant recipients.
Development of CMX001 (Brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses.[Pubmed:25120093]
Expert Rev Anti Infect Ther. 2014 Oct;12(10):1171-8.
CMX001 (hexadecyloxypropyl-cidofovir, Brincidofovir) is a broad spectrum, lipid conjugate of cidofovir that is converted intracellularly into the active antiviral, cidofovir diphosphate. The lipid conjugation results in oral bioavailability, higher intracellular concentrations of active drug, lower plasma concentrations of cidofovir and increased antiviral potency against dsDNA viruses.
Brincidofovir (CMX001) inhibits BK polyomavirus replication in primary human urothelial cells.[Pubmed:25801568]
Antimicrob Agents Chemother. 2015;59(6):3306-16.
BK polyomavirus (BKPyV)-associated hemorrhagic cystitis (PyVHC) complicates 5 to 15% of allogeneic hematopoietic stem cell transplantations. Targeted antivirals are still unavailable. Brincidofovir (BCV; previously CMX001) has shown inhibitory activity against diverse viruses, including BKPyV in a primary human renal tubule cell culture model of polyomavirus-associated nephropathy. We investigated the effects of BCV in BKPyV-infected and uninfected primary human urothelial cells (HUCs), the target cells of BKPyV in PyVHC. The BCV concentrations causing 50 and 90% reductions (EC50 and EC90) in the number of intracellular BKPyV genome equivalents per cell (icBKPyV) were 0.27 muM and 0.59 muM, respectively. At 0.63 muM, BCV reduced viral late gene expression by 90% and halted progeny release. Preinfection treatment for only 24 h reduced icBKPyV similarly to treatment from 2 to 72 h postinfection, while combined pre- and postinfection treatment suppressed icBKPyV completely. After investigating BCV's effects on HUC viability, mean selectivity indices at 50 and 90% inhibition (SI50 and SI90) calculated for cellular DNA replication were 2.7 and 2.9, respectively, those for mitochondrial activity were 8.9 and 10.4, those for total ATP were 8.6 and 8.2, and those for membrane integrity were 25.9 and 16.7. The antiviral and cytostatic effects, but less so the cytotoxic effects, were inversely related to cell density. The cytotoxic effects at concentrations of >/=10 muM were rapid and likely related to BCV's lipid moiety. After carefully defining the antiviral, cytostatic, and cytotoxic properties of BCV in HUCs, we conclude that a preemptive or prophylactic approach in PyVHC is likely to give the best results.
Co-administration of the broad-spectrum antiviral, brincidofovir (CMX001), with smallpox vaccine does not compromise vaccine protection in mice challenged with ectromelia virus.[Pubmed:25128688]
Antiviral Res. 2014 Nov;111:42-52.
Natural orthopoxvirus outbreaks such as vaccinia, cowpox, cattlepox and buffalopox continue to cause morbidity in the human population. Monkeypox virus remains a significant agent of morbidity and mortality in Africa. Furthermore, monkeypox virus's broad host-range and expanding environs make it of particular concern as an emerging human pathogen. Monkeypox virus and variola virus (the etiological agent of smallpox) are both potential agents of bioterrorism. The first line response to orthopoxvirus disease is through vaccination with first-generation and second-generation vaccines, such as Dryvax and ACAM2000. Although these vaccines provide excellent protection, their widespread use is impeded by the high level of adverse events associated with vaccination using live, attenuated virus. It is possible that vaccines could be used in combination with antiviral drugs to reduce the incidence and severity of vaccine-associated adverse events, or as a preventive in individuals with uncertain exposure status or contraindication to vaccination. We have used the intranasal mousepox (ectromelia) model to evaluate the efficacy of vaccination with Dryvax or ACAM2000 in conjunction with treatment using the broad spectrum antiviral, brincidofovir (BCV, CMX001). We found that co-treatment with BCV reduced the severity of vaccination-associated lesion development. Although the immune response to vaccination was quantifiably attenuated, vaccination combined with BCV treatment did not alter the development of full protective immunity, even when administered two days following ectromelia challenge. Studies with a non-replicating vaccine, ACAM3000 (MVA), confirmed that BCV's mechanism of attenuating the immune response following vaccination with live virus was, as expected, by limiting viral replication and not through inhibition of the immune system. These studies suggest that, in the setting of post-exposure prophylaxis, co-administration of BCV with vaccination should be considered a first response to a smallpox emergency in subjects of uncertain exposure status or as a means of reduction of the incidence and severity of vaccine-associated adverse events.


