N1,N12-Diethylspermine tetrahydrochloridePolyamine synthase inhibitor CAS# 113812-15-0 |
- SU14813
Catalog No.:BCC1971
CAS No.:627908-92-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 113812-15-0 | SDF | Download SDF |
| PubChem ID | 14056749 | Appearance | Powder |
| Formula | C14H38Cl4N4 | M.Wt | 404.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | N,N'-bis[3-(ethylamino)propyl]butane-1,4-diamine;tetrahydrochloride | ||
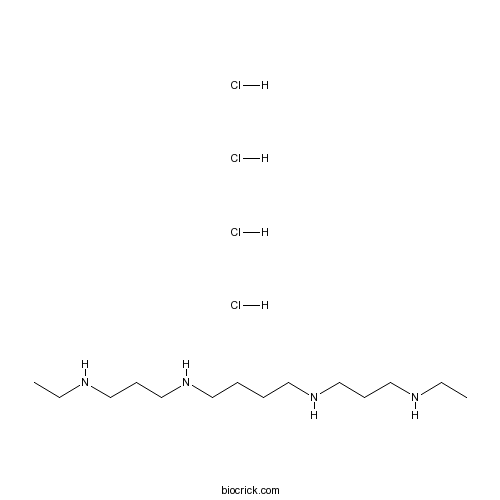
| SMILES | CCNCCCNCCCCNCCCNCC.Cl.Cl.Cl.Cl | ||
| Standard InChIKey | SCYOBNRPMHJGLB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H34N4.4ClH/c1-3-15-11-7-13-17-9-5-6-10-18-14-8-12-16-4-2;;;;/h15-18H,3-14H2,1-2H3;4*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Powerful antineoplastic agent in cultured cells and animal tumors (IC50 = 0.2 μM in L1210 cells). Inhibitor of polyamine synthases. Suppressor of mitochondrial DNA synthesis. |

N1,N12-Diethylspermine tetrahydrochloride Dilution Calculator

N1,N12-Diethylspermine tetrahydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4735 mL | 12.3674 mL | 24.7347 mL | 49.4694 mL | 61.8368 mL |
| 5 mM | 0.4947 mL | 2.4735 mL | 4.9469 mL | 9.8939 mL | 12.3674 mL |
| 10 mM | 0.2473 mL | 1.2367 mL | 2.4735 mL | 4.9469 mL | 6.1837 mL |
| 50 mM | 0.0495 mL | 0.2473 mL | 0.4947 mL | 0.9894 mL | 1.2367 mL |
| 100 mM | 0.0247 mL | 0.1237 mL | 0.2473 mL | 0.4947 mL | 0.6184 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Picrasidine T
Catalog No.:BCN6017
CAS No.:113808-03-0
- Z-Gly-OH
Catalog No.:BCC2770
CAS No.:1138-80-3
- (Z)-FeCP-oxindole
Catalog No.:BCC6079
CAS No.:1137967-28-2
- TAK960
Catalog No.:BCC6411
CAS No.:1137868-52-0
- MDL 72832 hydrochloride
Catalog No.:BCC6637
CAS No.:113777-40-5
- Dexmedetomidine
Catalog No.:BCC4326
CAS No.:113775-47-6
- Eudesm-4(15)-ene-3alpha,11-diol
Catalog No.:BCN4060
CAS No.:113773-90-3
- Ilexhainanoside D
Catalog No.:BCN7863
CAS No.:1137648-52-2
- LX1606
Catalog No.:BCC1713
CAS No.:1137608-69-5
- BRD 7552
Catalog No.:BCC8035
CAS No.:1137359-47-7
- cis-Ned 19
Catalog No.:BCC6089
CAS No.:1137264-00-6
- Tenatoprazole
Catalog No.:BCC4732
CAS No.:113712-98-4
- N-p-coumaroyl-N'-caffeoylputrescine
Catalog No.:BCN6018
CAS No.:1138156-77-0
- Cidofovir
Catalog No.:BCC2546
CAS No.:113852-37-2
- CX-5461
Catalog No.:BCC3700
CAS No.:1138549-36-6
- Cinnamyl 3-aminobut-2-enoate
Catalog No.:BCC8914
CAS No.:113898-97-8
- Caryophyllene oxide
Catalog No.:BCN6019
CAS No.:1139-30-6
- Koumine N-oxide
Catalog No.:BCN4807
CAS No.:113900-75-7
- PNU 74654
Catalog No.:BCC7704
CAS No.:113906-27-7
- PMPA (NMDA antagonist)
Catalog No.:BCC7308
CAS No.:113919-36-1
- Andrographidine C
Catalog No.:BCN4730
CAS No.:113963-39-6
- Andrographidine E
Catalog No.:BCN4729
CAS No.:113963-41-0
- (Z)-Akuammidine
Catalog No.:BCN6020
CAS No.:113973-31-2
- Cefozopran hydrochloride
Catalog No.:BCC8909
CAS No.:113981-44-5
Synthesis and biological evaluation of aminopolyamines.[Pubmed:15857111]
J Med Chem. 2005 May 5;48(9):3099-102.
Exploitation of the polyamine backbone as a vector for intracellular transport of various pharmacophores has focused largely on fixing the cargo molecule to one of the nitrogens in the linear chain. This communication describes the assembly of a model aminopolyamine analogue, 6-amino-N(1),N(12)-diethylspermine, and its biological properties. This amino polyamine presents an additional site of attachment for cargo molecules, reduces cell growth, and achieves cellular concentrations that are higher than those of N(1),N(12)-diethylspermine.
Selective cellular depletion of mitochondrial DNA by the polyamine analog N1,N12-bis(ethyl)spermine and its relationship to polyamine structure and function.[Pubmed:2017149]
Mol Pharmacol. 1991 Apr;39(4):487-94.
N1,N8-Bis(ethyl)spermidine (BESPD) and N1,N12-bis(ethyl)spermine (BESPM) are minimally modified analogs of spermidine and spermine that deplete cellular polyamine pools by suppressing key polyamine biosynthetic enzymes. The consequences of polyamine depletion and the concomitant analog replacement of these pools were compared on two cellular DNA targets, mitochondrial DNA (mtDNA) and a defined nuclear DNA episome present in 935.1 mouse fibroblasts. The spermidine analog, BESPD, depleted cellular putrescine and spermidine pools, but not spermine pools, and had no effect on either DNA target. Treatment with the corresponding analog of spermine, BESPM, resulted in a near-total depletion of all three polyamine pools and a greater than 80% reduction in the cellular content of mtDNA, without affecting the levels of the nuclear episome. Topological forms analysis by Southern blotting of mtDNA and episomal DNA from BESPM-treated cells failed to reveal any forms interconversion, indicating the absence of analog-induced single- or double-strand break damage to either DNA target. The growth-dependent loss of mtDNA is consistent with a rapid cessation of mtDNA replication and subsequent dilution of existing mtDNA copies by cell division. Similar decreases in polyamine pools and mtDNA were also observed in L1210 cells treated with BESPM. When a comparable level of polyamine depletion was produced in L1210 cells by specific enzyme inhibitors, there was no effect on the cellular content of mtDNA, and BESPD was not rendered capable of decreasing mtDNA levels. Because the analogs are structurally similar to the naturally occurring polyamines and would be expected to have similar binding properties, the loss in mtDNA may reflect dysfunctional replacement by BESPM at spermine-specific binding sites in the mitochondrion.
Combined regulation of ornithine and S-adenosylmethionine decarboxylases by spermine and the spermine analogue N1 N12-bis(ethyl)spermine.[Pubmed:2344358]
Biochem J. 1990 May 15;268(1):207-12.
In the present study, the spermine (SPM) analogue N1N12-bis(ethyl)spermine (BESPM) is compared with SPM in its ability to regulate ornithine decarboxylase (ODC) and S-adenosyl-L-methionine decarboxylase (AdoMetDC) activities in intact L1210 cells and in the mechanism(s) by which this is accomplished. Unlike the comparable spermidine (SPD) analogue N1N8-bis(ethyl)spermidine, which regulates only ODC, BESPM suppresses both ODC and AdoMetDC activities. With 1 microM-SPM or -BESPM, near-maximal suppression of enzyme activity (i.e. less than 70%) was achieved after 2 h for ODC and 12 h for AdoMetDC. After such treatment, ODC activity fully recovered within 2-4 h, and that of AdoMetDC within 12 h, when cells were reseeded into drug-free media. It was deduced that an intracellular accumulation of BESPM or SPM equivalent to only approximately 200-450 pmol/10(6) cells was sufficient to fully invoke ODC regulatory mechanisms. Decreases in both enzyme activities after BESPM or SPM treatment were closely paralleled by concomitant decreases in the amount of enzyme protein. Since cellular ODC or AdoMetDC mRNA was not similarly decreased by either BESPM or SPM treatment, it was concluded that translational and/or post-translational mechanisms were probably responsible for enzyme regulation. In support of the former of these possibilities, it was demonstrated that both BESPM and SPM preferentially inhibited the translation in vitro of ODC and AdoMetDC relative to albumin in a reticulocyte-lysate system. On the basis of the consistent similarities between BESPM and SPM in all parameters studied, it is concluded that the analogue most likely acts by mechanisms identical with those by which SPM acts in suppressing polyamine biosynthesis.
Synthetic polyamine analogues as antineoplastics.[Pubmed:3373487]
J Med Chem. 1988 Jun;31(6):1183-90.
In this paper, we report on the synthesis and biological activity of a number of N-alkylated spermine compounds. The dialkylspermines N1,N12-dimethylspermine (DMSPM-2), N1,N12-diethylspermine (DESPM-3), and N1,N12-dipropylspermine (DPSPM-4) are all shown to inhibit the growth of L1210 cells in culture with IC50 values of less than 1 microM at 96 h. Furthermore, DESPM-3 is shown to be similarly active against Daudi and HL-60 cells in culture. A structure-activity relationship is shown to exist between the position at which spermine is alkylated and its antiproliferative properties. The activity of 10 microM DESPM-3 against L1210 cells was shown to be cytostatic, with greater than 90% cell viability by trypan blue exclusion, even after a 144-h exposure. When L1210 cells were treated with 10 microM DESPM-3 over a 144-h period, their size and mitochondrial DNA content were gradually but substantially diminished. However, flow cytometric measurements of the nuclear DNA content of these treated cells at 96 h indicated only slightly reduced S and G2 populations and significant changes only after 144 h. A cloning assay performed on the cells after 96 h of exposure to this drug (10 microM) indicated that the cells were not growing. Finally, when male DBA/2 mice, inoculated with L1210 leukemia cells, were treated with DESPM-3, their life span was increased in excess of 200% relative to untreated controls. Moreover, many long-term survivors were apparently tumor free at the end of the experiment (60 days).


