Cefozopran hydrochlorideCAS# 113981-44-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 113981-44-5 | SDF | Download SDF |
| PubChem ID | 5491954 | Appearance | Powder |
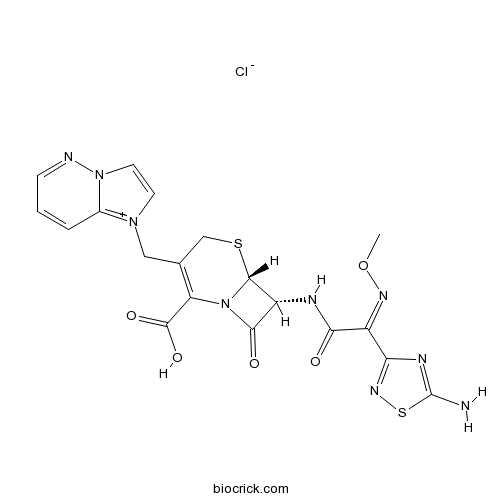
| Formula | C19H18ClN9O5S2 | M.Wt | 552.0 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SCE-2787 hydrochloride | ||
| Solubility | H2O : ≥ 52 mg/mL (94.20 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (6R,7R)-7-[[(2Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-methoxyiminoacetyl]amino]-3-(imidazo[1,2-b]pyridazin-4-ium-1-ylmethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;chloride | ||
| SMILES | CON=C(C1=NSC(=N1)N)C(=O)NC2C3N(C2=O)C(=C(CS3)CN4C=C[N+]5=C4C=CC=N5)C(=O)O.[Cl-] | ||
| Standard InChIKey | NTJHUKMPVIFDNY-XFDPNJHTSA-N | ||
| Standard InChI | InChI=1S/C19H17N9O5S2.ClH/c1-33-24-11(14-23-19(20)35-25-14)15(29)22-12-16(30)28-13(18(31)32)9(8-34-17(12)28)7-26-5-6-27-10(26)3-2-4-21-27;/h2-6,12,17H,7-8H2,1H3,(H3-,20,22,23,25,29,31,32);1H/b24-11-;/t12-,17-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cefozopran hydrochloride Dilution Calculator

Cefozopran hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8116 mL | 9.058 mL | 18.1159 mL | 36.2319 mL | 45.2899 mL |
| 5 mM | 0.3623 mL | 1.8116 mL | 3.6232 mL | 7.2464 mL | 9.058 mL |
| 10 mM | 0.1812 mL | 0.9058 mL | 1.8116 mL | 3.6232 mL | 4.529 mL |
| 50 mM | 0.0362 mL | 0.1812 mL | 0.3623 mL | 0.7246 mL | 0.9058 mL |
| 100 mM | 0.0181 mL | 0.0906 mL | 0.1812 mL | 0.3623 mL | 0.4529 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cefozopran Hcl(SCE 2787 Hcl) is a fourth-generation cephalosporin. Target: Antibacterial Cefozopran is a fourth-generation cephalosporin.
References:
[1]. http://www.toku-e.com/Assets/MIC/Cefozopran%20hydrochloride.pdf
- (Z)-Akuammidine
Catalog No.:BCN6020
CAS No.:113973-31-2
- Andrographidine E
Catalog No.:BCN4729
CAS No.:113963-41-0
- Andrographidine C
Catalog No.:BCN4730
CAS No.:113963-39-6
- PMPA (NMDA antagonist)
Catalog No.:BCC7308
CAS No.:113919-36-1
- PNU 74654
Catalog No.:BCC7704
CAS No.:113906-27-7
- Koumine N-oxide
Catalog No.:BCN4807
CAS No.:113900-75-7
- Caryophyllene oxide
Catalog No.:BCN6019
CAS No.:1139-30-6
- Cinnamyl 3-aminobut-2-enoate
Catalog No.:BCC8914
CAS No.:113898-97-8
- CX-5461
Catalog No.:BCC3700
CAS No.:1138549-36-6
- Cidofovir
Catalog No.:BCC2546
CAS No.:113852-37-2
- N-p-coumaroyl-N'-caffeoylputrescine
Catalog No.:BCN6018
CAS No.:1138156-77-0
- N1,N12-Diethylspermine tetrahydrochloride
Catalog No.:BCC6669
CAS No.:113812-15-0
- 5-Hydroxy-7,8-dimethoxyflavanone
Catalog No.:BCN6021
CAS No.:113981-49-0
- Golgicide A
Catalog No.:BCC4373
CAS No.:1139889-93-2
- Erythromycin
Catalog No.:BCC4778
CAS No.:114-07-8
- Scopolamine hydrobromide
Catalog No.:BCN1199
CAS No.:114-49-8
- Neostigmine Bromide
Catalog No.:BCC4563
CAS No.:114-80-7
- Phenformin
Catalog No.:BCC9120
CAS No.:114-86-3
- Phaclofen
Catalog No.:BCC6562
CAS No.:114012-12-3
- 16-Epivoacarpine
Catalog No.:BCN3940
CAS No.:114027-38-2
- Humantenidine
Catalog No.:BCN4754
CAS No.:114027-39-3
- Ibandronic acid
Catalog No.:BCC5204
CAS No.:114084-78-5
- Cabozantinib malate (XL184)
Catalog No.:BCC4388
CAS No.:1140909-48-3
- Tirandalydigin
Catalog No.:BCN1860
CAS No.:114118-91-1
DEVELOPMENT AND VALIDATION OF THE STABILITY-INDICATING LC-UV METHOD FOR DETERMINATION OF CEFOZOPRAN HYDROCHLORIDE.[Pubmed:26642650]
Acta Pol Pharm. 2015 May-Jun;72(3):423-7.
The stability-indicating LC assay method was developed and validated for quantitative determination of Cefozopran hydrochloride (CZH) in the presence of degradation products formed during the forced degradation studies. An isocratic, RP-HPLC method was developed with C-18 (250 mm x 4.6 mm, 5 microm) column and 12 mM ammonium acetate-acetonitrile (92:8, v/v) as a mobile phase. The flow rate of the mobile phase was 1.0 mL/min. Detection wavelength was 260 not and temperature was 30 degrees C. Cefozopran hydrochloride as other cephalosporins was subjected to stress conditions of degradation in aqueous solutions including hydrolysis, oxidation, photolysis and thermal degradation. The developed method was validated with regard to linearity, accuracy, precision, selectivity and robustness. The method was applied successfully for identification and determination of Cefozopran hydrochloride in pharmaceuticals and during kinetic studies.
[Pharmacokinetics of injected cefozopran hydrochloride in healthy volunteers].[Pubmed:23230745]
Sichuan Da Xue Xue Bao Yi Xue Ban. 2012 Sep;43(5):711-4.
OBJECTIVE: To study the pharmacokinetics of injected Cefozopran hydrochloride in healthy volunteers. METHODS: 24 healthy volunteers were enrolled to receive low (0.5 g), middle (1.0 g), high (2.0 g) doses of single injection and multiple doses (1.0 g) injection of Cefozopran hydrochloride in an open randomized study. The plasma concentrations of cefozopran were determined by RP-HPLC. The DAS2.0 was used to fit the concentration-time data and to calculate the pharmacokinetic parameters. RESULTS: The main pharmaeokinetic parameters for a single injection of low, middle and high doses of cefozopran were as follows: Cmax (48.27 +/- 9.84), (77.99 +/- 15.08) and (171.59 +/- 18.27) mg/L; Tmax (0.50 +/- 0.00), (0.51 +/- 0.02) and (0.51 + 0.02) h; AUCo-t (92.43 +/- 24.02), (152.45 +/- 16.26) and (341.03 +/- 44.16) mg x h/L; t1/2beta (1.97 +/- 0.19), (2.44 +/- 0.24) and (2.18 +/- 0.31) h, respectively. The main pharmacokinetic parameters for a multiple doses injection of cefozopran were as follows: Cmax (80.39 +/- 11.86) mg/L; Tmax (0.51 +/- 0.02) h; AUCo-t (159.74 +/- 15.06) mg x h/L; t1/2beta (2.55 +/- 0.55) h. The accumulative rate of cefozopran through urine pathway within 24 h was (89.4 +/- 15.5)%. The statistical analysis showed that Cmax, AUCo-t, and AUCo-infinity increased significantly with increased doses of injection (P < 0.05). Those parameters were linearly correlated with the doses of injection (r = 0.9950, 0.9960, 0.9963). However, dosage did not have an impact on other pharmacokinetic parameters (P > 0.05). No gender differences in the parameters were found (P > 0.05). CONCLUSION: Cefozopran hydrochloride performs a linear kinetics in healthy volunteers. The main pharmacokinetic parameters have no significant gender differences, and there is no drug accumulated with multiple doses of injection.


