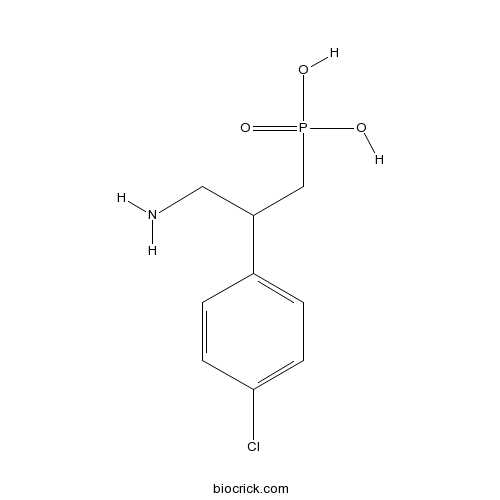
PhaclofenWeak, selective GABAB antagonist CAS# 114012-12-3 |
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- Roscovitine (Seliciclib,CYC202)
Catalog No.:BCC1105
CAS No.:186692-46-6
- Nu 6027
Catalog No.:BCC1154
CAS No.:220036-08-8
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- AT7519 Hydrochloride
Catalog No.:BCC1376
CAS No.:902135-91-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 114012-12-3 | SDF | Download SDF |
| PubChem ID | 1641 | Appearance | Powder |
| Formula | C9H13ClNO3P | M.Wt | 249.63 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in 1eq. NaOH | ||
| Chemical Name | [3-amino-2-(4-chlorophenyl)propyl]phosphonic acid | ||
| SMILES | C1=CC(=CC=C1C(CN)CP(=O)(O)O)Cl | ||
| Standard InChIKey | VSGNGLJPOGUDON-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H13ClNO3P/c10-9-3-1-7(2-4-9)8(5-11)6-15(12,13)14/h1-4,8H,5-6,11H2,(H2,12,13,14) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective GABAB antagonist. Phosphono analog of GABAB agonist baclofen. |

Phaclofen Dilution Calculator

Phaclofen Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0059 mL | 20.0296 mL | 40.0593 mL | 80.1186 mL | 100.1482 mL |
| 5 mM | 0.8012 mL | 4.0059 mL | 8.0119 mL | 16.0237 mL | 20.0296 mL |
| 10 mM | 0.4006 mL | 2.003 mL | 4.0059 mL | 8.0119 mL | 10.0148 mL |
| 50 mM | 0.0801 mL | 0.4006 mL | 0.8012 mL | 1.6024 mL | 2.003 mL |
| 100 mM | 0.0401 mL | 0.2003 mL | 0.4006 mL | 0.8012 mL | 1.0015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Phenformin
Catalog No.:BCC9120
CAS No.:114-86-3
- Neostigmine Bromide
Catalog No.:BCC4563
CAS No.:114-80-7
- Scopolamine hydrobromide
Catalog No.:BCN1199
CAS No.:114-49-8
- Erythromycin
Catalog No.:BCC4778
CAS No.:114-07-8
- Golgicide A
Catalog No.:BCC4373
CAS No.:1139889-93-2
- 5-Hydroxy-7,8-dimethoxyflavanone
Catalog No.:BCN6021
CAS No.:113981-49-0
- Cefozopran hydrochloride
Catalog No.:BCC8909
CAS No.:113981-44-5
- (Z)-Akuammidine
Catalog No.:BCN6020
CAS No.:113973-31-2
- Andrographidine E
Catalog No.:BCN4729
CAS No.:113963-41-0
- Andrographidine C
Catalog No.:BCN4730
CAS No.:113963-39-6
- PMPA (NMDA antagonist)
Catalog No.:BCC7308
CAS No.:113919-36-1
- PNU 74654
Catalog No.:BCC7704
CAS No.:113906-27-7
- 16-Epivoacarpine
Catalog No.:BCN3940
CAS No.:114027-38-2
- Humantenidine
Catalog No.:BCN4754
CAS No.:114027-39-3
- Ibandronic acid
Catalog No.:BCC5204
CAS No.:114084-78-5
- Cabozantinib malate (XL184)
Catalog No.:BCC4388
CAS No.:1140909-48-3
- Tirandalydigin
Catalog No.:BCN1860
CAS No.:114118-91-1
- Kuguacin J
Catalog No.:BCN3055
CAS No.:1141453-65-7
- Kuguacin N
Catalog No.:BCN3056
CAS No.:1141453-73-7
- Butyraxanthone B
Catalog No.:BCN3603
CAS No.:1141754-81-5
- Z-Ala-OH
Catalog No.:BCC3055
CAS No.:1142-20-7
- PAOPA
Catalog No.:BCC6353
CAS No.:114200-31-6
- Puerarin 6''-O-xyloside
Catalog No.:BCN2780
CAS No.:114240-18-5
- CI 976
Catalog No.:BCC7299
CAS No.:114289-47-3
Effects of baclofen and phaclofen on receptive field properties of rat whisker barrel neurons.[Pubmed:8814908]
Brain Res. 1996 Mar 18;712(2):325-8.
Extracellular single-unit recordings were made in somatosensory cortical barrels of fentanyl-sedated rats. Whiskers were deflected singly or in paired combinations. Iontophoretically-applied (-)-baclofen disproportionately reduced weak responses, and Phaclofen disproportionately increased them, resulting in more tightly focused or more broadly focused receptive fields, respectively. Both drugs had only minor effects on surround inhibition. In light of previous findings, we conclude that GABAA and GABAB mechanisms both act to enhance spatial contrast, but that the former plays a much greater role in enhancing temporal resolution.
Delayed response deficits induced by local injection of GABA antagonists (bicuculline and phaclofen) into area 46 in infant rhesus monkeys.[Pubmed:8815445]
Neurosci Res. 1996 Feb;24(3):245-63.
In freely moving infant rhesus monkeys, a small amount of GABAa or GABAb antagonist (bicuculline methiodide (BMI) or Phaclofen (PHAC)) was injected into the left- or right-side of Walker's area 46 (BMI, left-side 18 sites, right-side 15 sites; PHAC, left-side six sites, right-side five sites). Deficits in the performance of a 5-s delayed response task were then studied. Regardless of which side of the brain was injected, the correct performance rate was reduced for the hand used most often. An increase in the number of error trials was seen both in the primary, most used and non-primary, less used hands. In addition, the number of perseverative errors, using the primary and non-primary hands, also increased. BMI and PHAC produced similar reductions in performance and increased perseveration. With BMI, no clear difference was observed in performance reduction between the left- and right-side injections, while a difference was observed in the increase in the number of perseverative errors. In monkeys that primarily used their right hands, the right-side BMI injections induced more perseverative errors to the left position with the right hand, and left-side injections induced perseverative errors to both the left and right positions with both the left and right hands. In monkeys that primarily used their left hands, right-side BMI injections induced more perseverative errors to the left position with both the left and right hands and left-side injections induced, as seen in the right hand users, perseverative errors to both the left and right positions with both the left and right hands. Such lateral differences were not observed with PHAC. These results suggest that both GABAa and GABAb inhibition of area 46 are involved in the correct performance of a 5-s delayed response task.
Altered GABAB receptor immunoreactivity in the gerbil hippocampus induced by baclofen and phaclofen, not seizure activity.[Pubmed:15236866]
Neurosci Res. 2004 Aug;49(4):405-16.
The present study was performed to determine whether the effects induced by GABA(B) receptor-acting drugs would be related with the alteration in GABA(B) receptor expression in the hippocampus using Mongolian gerbil, a genetic epilepsy model. The distribution patterns of both GABA(B) receptor 1A/B and GABA(B)receptor 2 immunoreactivities were similarly detected in the hippocampi of normal and seizure-prone gerbils. Following baclofen (GABA(B) receptor agonist) or Phaclofen (GABA(B) receptor antagonist) treatment, GABA(B) receptor immunoreactivities were decreased or increased by dose-dependent manners, respectively. Vigabatrin (GABA transaminase inhibitor) or 3-mercaptopropionic acid (GAD inhibitor) treatment did not affect GABA(B) receptor expressions. These findings suggest that GABA(B) receptor expression in the gerbil hippocampus may be altered by baclofen or Phaclofen treatment.
The human GABA(B1b) and GABA(B2) heterodimeric recombinant receptor shows low sensitivity to phaclofen and saclofen.[Pubmed:11082110]
Br J Pharmacol. 2000 Nov;131(6):1050-4.
1. The aim of this study was to characterize the pharmacological profile of the GABA(B1)/GABA(B2) heterodimeric receptor expressed in Chinese hamster ovary (CHO) cells. We have compared receptor binding affinity and functional activity for a series of agonists and antagonists. 2. The chimeric G-protein, G(qi5), was used to couple receptor activation to increases in intracellular calcium for functional studies on the Fluorimetric Imaging Plate Reader (FLIPR), using a stable GABA(B1)/GABA(B2)/G(qi5) CHO cell line. [(3)H]-CGP-54626 was used in radioligand binding studies in membranes prepared from the same cell line. 3. The pharmacological profile of the recombinant GABA(B1/B2) receptor was consistent with that of native GABA(B) receptors in that it was activated by GABA and baclofen and inhibited by CGP-54626A and SCH 50911. 4. Unlike native receptors, the GABA(B1)/GABA(B2)/G(qi5) response was not inhibited by high microMolar concentration of Phaclofen, saclofen or CGP 35348. 5. This raises the possibility that the GABA(B1)/GABA(B2)/G(qi5) recombinant receptor may represent the previously described GABA(B) receptor subtype which is relatively resistant to inhibition by Phaclofen.
Comparison of antagonism by phaclofen of baclofen induced hyperpolarizations and synaptically mediated late hyperpolarizing potentials recorded intracellularly from rat dorsolateral septal neurons.[Pubmed:3362432]
Neurosci Lett. 1988 Mar 21;86(1):77-81.
Phaclofen has recently been described as an antagonist to baclofen at both peripheral and central receptors. We have applied Phaclofen in known concentrations to an isolated rat brain slice preparation containing the septal nuclei. Our data demonstrate that Phaclofen antagonizes responses to exogenously applied baclofen in a competitive manner. On the other hand, Phaclofen is not as effective in antagonizing competitively the synaptically mediated late hyperpolarizing response (LHP) recorded from the same or similar dorsolateral septal nucleus (DLSN) neurons from which baclofen responses were recorded. Our data support the usefulness of Phaclofen as a competitive antagonist of baclofen, and suggest that when larger stimulus intensities are applied, the LHP in the dorsolateral septum of the rat may be mediated by a transmitter in addition to gamma-aminobutyric acid (GABA).
Phaclofen: a peripheral and central baclofen antagonist.[Pubmed:3032346]
Brain Res. 1987 Mar 3;405(1):150-4.
Phaclofen, the phosphonic acid derivative of baclofen, reversibly antagonized the depression of the cholinergic twitch response of the guinea pig ileum and distal colon by either baclofen or GABA. When administered microelectrophoretically, Phaclofen reversibly blocked the presumed presynaptic reduction by baclofen of the monosynaptic excitation of spinal interneurones by impulses in primary afferent fibres of the cat but did not block the postsynaptic depressant action of baclofen on these neurones. Phaclofen may thus be useful in determining the physiological significance of central and peripheral bicuculline-insensitive receptors with which GABA and (-)-baclofen interact.


