PF-8380Autotaxin inhibitor,potent and specific CAS# 1144035-53-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1144035-53-9 | SDF | Download SDF |
| PubChem ID | 25265312 | Appearance | Powder |
| Formula | C22H21Cl2N3O5 | M.Wt | 478.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 6.67 mg/mL (13.94 mM; ultrasonic and adjust pH to 6 with HCl) | ||
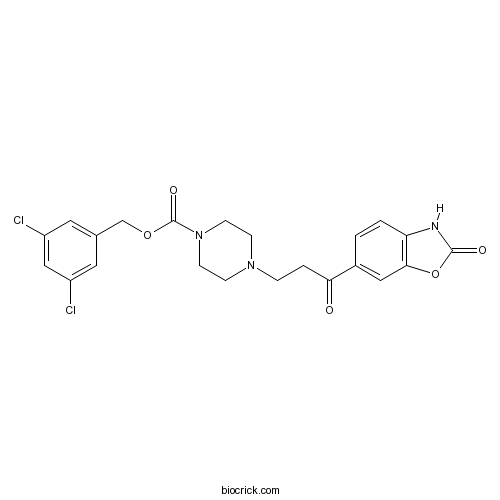
| Chemical Name | (3,5-dichlorophenyl)methyl 4-[3-oxo-3-(2-oxo-3H-1,3-benzoxazol-6-yl)propyl]piperazine-1-carboxylate | ||
| SMILES | C1CN(CCN1CCC(=O)C2=CC3=C(C=C2)NC(=O)O3)C(=O)OCC4=CC(=CC(=C4)Cl)Cl | ||
| Standard InChIKey | JMSUDQYHPSNBSN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H21Cl2N3O5/c23-16-9-14(10-17(24)12-16)13-31-22(30)27-7-5-26(6-8-27)4-3-19(28)15-1-2-18-20(11-15)32-21(29)25-18/h1-2,9-12H,3-8,13H2,(H,25,29) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent autotaxin inhibitor (IC50 = 2.8 nM in isolated enzyme assay; 101 nM in human whole blood). Modulates lysophosphatidic acid (LPA) levels in vivo and in vitro by directly inhibiting autotaxin; reduces LPA levels both in plasma and at site of inflammation. Decreases leptin-induced MMP13 expression, in vitro. Orally available. |

PF-8380 Dilution Calculator

PF-8380 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0906 mL | 10.453 mL | 20.9061 mL | 41.8121 mL | 52.2652 mL |
| 5 mM | 0.4181 mL | 2.0906 mL | 4.1812 mL | 8.3624 mL | 10.453 mL |
| 10 mM | 0.2091 mL | 1.0453 mL | 2.0906 mL | 4.1812 mL | 5.2265 mL |
| 50 mM | 0.0418 mL | 0.2091 mL | 0.4181 mL | 0.8362 mL | 1.0453 mL |
| 100 mM | 0.0209 mL | 0.1045 mL | 0.2091 mL | 0.4181 mL | 0.5227 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PF-8380 is a specific and potent inhibitor of Autotaxin (ATX) with an IC50 value of 2.8 nM [1].
Autotaxin (ATX) is a secreted enzyme having lysophospholipase D activity
- Fmoc-D-Leu-OH
Catalog No.:BCC3511
CAS No.:114360-54-2
- TAK 21d
Catalog No.:BCC5609
CAS No.:1143578-94-2
- AZD5363
Catalog No.:BCC1073
CAS No.:1143532-39-1
- Guanosine Hydrate
Catalog No.:BCC5326
CAS No.:1143525-19-2
- Soyacerebroside I
Catalog No.:BCN6022
CAS No.:114297-20-0
- CI 976
Catalog No.:BCC7299
CAS No.:114289-47-3
- Puerarin 6''-O-xyloside
Catalog No.:BCN2780
CAS No.:114240-18-5
- PAOPA
Catalog No.:BCC6353
CAS No.:114200-31-6
- Z-Ala-OH
Catalog No.:BCC3055
CAS No.:1142-20-7
- Butyraxanthone B
Catalog No.:BCN3603
CAS No.:1141754-81-5
- Kuguacin N
Catalog No.:BCN3056
CAS No.:1141453-73-7
- Kuguacin J
Catalog No.:BCN3055
CAS No.:1141453-65-7
- WYE-125132 (WYE-132)
Catalog No.:BCC4608
CAS No.:1144068-46-1
- Beta-Furoyleupatolide
Catalog No.:BCN6407
CAS No.:114437-24-0
- BNP (1-32), human
Catalog No.:BCC1039
CAS No.:114471-18-0
- Oroxin B
Catalog No.:BCN1203
CAS No.:114482-86-9
- Z-Ser-OH
Catalog No.:BCC2743
CAS No.:1145-80-8
- (-)-U-50488 hydrochloride
Catalog No.:BCC6666
CAS No.:114528-79-9
- (+)-U-50488 hydrochloride
Catalog No.:BCC6656
CAS No.:114528-81-3
- Spiramine A
Catalog No.:BCN6023
CAS No.:114531-28-1
- Honyucitrin
Catalog No.:BCN4728
CAS No.:114542-44-8
- Regelidine
Catalog No.:BCN3094
CAS No.:114542-54-0
- Galanin (1-29) (rat, mouse)
Catalog No.:BCC5928
CAS No.:114547-31-8
- Boeravinone B
Catalog No.:BCN6466
CAS No.:114567-34-9
PF-8380 and closely related analogs: synthesis and structure-activity relationship towards autotaxin inhibition and glioma cell viability.[Pubmed:23300119]
Arch Pharm (Weinheim). 2013 Feb;346(2):91-7.
A series of PF-8380 analogs, a recently developed autotaxin inhibitor, was explored. Inhibition of autotaxin by these analogs, as well as by all PF-8380 synthetic intermediates, shows the importance of meta-dichlorobenzyl and benzo[d]oxazol-2(3H)-one fragments. However, analogs 8 and 9, bearing only the benzo[d]oxazol-2(3H)-one moiety, are more cytotoxic on the LN229 glioblastoma cell line than PF-8380 and temozolomide (TMZ).
Autotaxin Inhibition with PF-8380 Enhances the Radiosensitivity of Human and Murine Glioblastoma Cell Lines.[Pubmed:24062988]
Front Oncol. 2013 Sep 17;3:236.
PURPOSE: Glioblastoma multiforme (GBM) is an aggressive primary brain tumor that is radio-resistant and recurs despite aggressive surgery, chemo, and radiotherapy. Autotaxin (ATX) is over expressed in various cancers including GBM and is implicated in tumor progression, invasion, and angiogenesis. Using the ATX specific inhibitor, PF-8380, we studied ATX as a potential target to enhance radiosensitivity in GBM. METHODS AND MATERIALS: Mouse GL261 and Human U87-MG cells were used as GBM cell models. Clonogenic survival assays and tumor transwell invasion assays were performed using PF-8380 to evaluate role of ATX in survival and invasion. Radiation dependent activation of Akt was analyzed by immunoblotting. Tumor induced angiogenesis was studied using the dorsal skin fold model in GL261. Heterotopic mouse GL261 tumors were used to evaluate the efficacy of PF-8380 as a radiosensitizer. RESULTS: Pre-treatment of GL261 and U87-MG cells with 1 muM PF-8380 followed by 4 Gy irradiation resulted in decreased clonogenic survival, decreased migration (33% in GL261; P = 0.002 and 17.9% in U87-MG; P = 0.012), decreased invasion (35.6% in GL261; P = 0.0037 and 31.8% in U87-MG; P = 0.002), and attenuated radiation-induced Akt phosphorylation. In the tumor window model, inhibition of ATX abrogated radiation induced tumor neovascularization (65%; P = 0.011). In a heterotopic mouse GL261 tumors untreated mice took 11.2 days to reach a tumor volume of 7000 mm(3), however combination of PF-8380 (10 mg/kg) with irradiation (five fractions of 2 Gy) took more than 32 days to reach a tumor volume of 7000 mm(3). CONCLUSION: Inhibition of ATX by PF-8380 led to decreased invasion and enhanced radiosensitization of GBM cells. Radiation-induced activation of Akt was abrogated by inhibition of ATX. Furthermore, inhibition of ATX led to diminished tumor vascularity and delayed tumor growth. These results suggest that inhibition of ATX may ameliorate GBM response to radiotherapy.
Autotaxin: its role in biology of melanoma cells and as a pharmacological target.[Pubmed:21423677]
Enzyme Res. 2011 Mar 8;2011:194857.
Autotaxin (ATX) is an extracellular lysophospholipase D (lysoPLD) released from normal cells and cancer cells. Activity of ATX is detected in various biological fluids. The lysophosphatidic acid (LPA) is the main product of ATX. LPA acting through specific G protein-coupled receptors (LPA(1)-LPA(6)) affects immunological response, normal development, and malignant tumors' formation and progression. In this review, the impact of autotoxin on biology of melanoma cells and potential treatment is discussed.
A novel autotaxin inhibitor reduces lysophosphatidic acid levels in plasma and the site of inflammation.[Pubmed:20392816]
J Pharmacol Exp Ther. 2010 Jul;334(1):310-7.
Autotaxin is the enzyme responsible for the production of lysophosphatidic acid (LPA) from lysophosphatidyl choline (LPC), and it is up-regulated in many inflammatory conditions, including but not limited to cancer, arthritis, and multiple sclerosis. LPA signaling causes angiogenesis, mitosis, cell proliferation, and cytokine secretion. Inhibition of autotaxin may have anti-inflammatory properties in a variety of diseases; however, this hypothesis has not been tested pharmacologically because of the lack of potent inhibitors. Here, we report the development of a potent autotaxin inhibitor, PF-8380 [6-(3-(piperazin-1-yl)propanoyl)benzo[d]oxazol-2(3H)-one] with an IC(50) of 2.8 nM in isolated enzyme assay and 101 nM in human whole blood. PF-8380 has adequate oral bioavailability and exposures required for in vivo testing of autotaxin inhibition. Autotaxin's role in producing LPA in plasma and at the site of inflammation was tested in a rat air pouch model. The specific inhibitor PF-8380, dosed orally at 30 mg/kg, provided >95% reduction in both plasma and air pouch LPA within 3 h, indicating autotaxin is a major source of LPA during inflammation. At 30 mg/kg PF-8380 reduced inflammatory hyperalgesia with the same efficacy as 30 mg/kg naproxen. Inhibition of plasma autotaxin activity correlated with inhibition of autotaxin at the site of inflammation and in ex vivo whole blood. Furthermore, a close pharmacokinetic/pharmacodynamic relationship was observed, which suggests that LPA is rapidly formed and degraded in vivo. PF-8380 can serve as a tool compound for elucidating LPA's role in inflammation.


