TAK-733MEK allosteric site inhibitor CAS# 1035555-63-5 |
- Trametinib (GSK1120212)
Catalog No.:BCC1282
CAS No.:871700-17-3
- Vemurafenib (PLX4032, RG7204)
Catalog No.:BCC1269
CAS No.:918504-65-1
- PLX-4720
Catalog No.:BCC1280
CAS No.:918505-84-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1035555-63-5 | SDF | Download SDF |
| PubChem ID | 24963252 | Appearance | Powder |
| Formula | C17H15F2IN4O4 | M.Wt | 504.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 33 mg/mL (65.45 mM) *"≥" means soluble, but saturation unknown. | ||
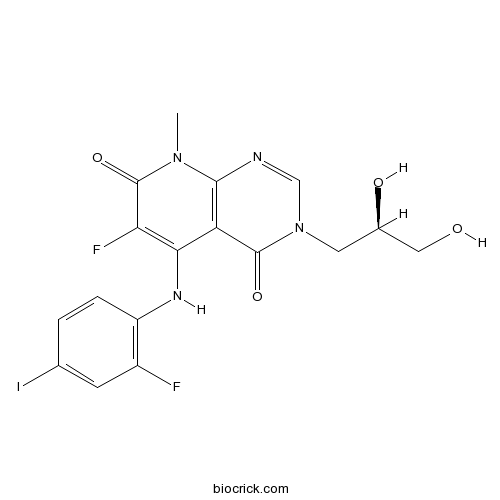
| Chemical Name | 3-[(2R)-2,3-dihydroxypropyl]-6-fluoro-5-(2-fluoro-4-iodoanilino)-8-methylpyrido[2,3-d]pyrimidine-4,7-dione | ||
| SMILES | CN1C2=C(C(=C(C1=O)F)NC3=C(C=C(C=C3)I)F)C(=O)N(C=N2)CC(CO)O | ||
| Standard InChIKey | RCLQNICOARASSR-SECBINFHSA-N | ||
| Standard InChI | InChI=1S/C17H15F2IN4O4/c1-23-15-12(16(27)24(7-21-15)5-9(26)6-25)14(13(19)17(23)28)22-11-3-2-8(20)4-10(11)18/h2-4,7,9,22,25-26H,5-6H2,1H3/t9-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | TAK-733 is a potent and selective inhibitor of MEK allosteric site for MEK1 with an IC50 value of 3.2 nM. | |||||
| Targets | MEK1 | |||||
| IC50 | 3.2 nM | |||||

TAK-733 Dilution Calculator

TAK-733 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9832 mL | 9.9161 mL | 19.8322 mL | 39.6644 mL | 49.5805 mL |
| 5 mM | 0.3966 mL | 1.9832 mL | 3.9664 mL | 7.9329 mL | 9.9161 mL |
| 10 mM | 0.1983 mL | 0.9916 mL | 1.9832 mL | 3.9664 mL | 4.9581 mL |
| 50 mM | 0.0397 mL | 0.1983 mL | 0.3966 mL | 0.7933 mL | 0.9916 mL |
| 100 mM | 0.0198 mL | 0.0992 mL | 0.1983 mL | 0.3966 mL | 0.4958 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
TAK-733 is a potent, ATP-noncompetitive and selective inhibitor of MEK allosteric site with the IC50 value of 3.2nM [1].
TAK-733 has been shown potent enzymatic and cell activity with an IC50 value of 3.2nM against constitutively active MEK enzyme and an EC50 of 1.9nM against ERK phosphorylation in cells. In addition, TAK-733 has also shown the low clearance and high oral bioavailability based on the pharmacokinetics of TAK-733 in all species (Mouse, rat, dog and Monkey). Furthermore, TAK-733 has been reported to broad inhibit tumor activity in mouse xenograft models of human cancer (melanoma, colorectal, NSCLC, pancreatic and breast cancer) [1].
References:
[1] Dong Q1, Dougan DR, Gong X, Halkowycz P, Jin B, Kanouni T, O'Connell SM, Scorah N, Shi L, Wallace MB, Zhou F. Discovery of TAK-733, a potent and selective MEK allosteric site inhibitor for the treatment of cancer. Bioorg Med Chem Lett. 2011 Mar 1;21(5):1315-9. doi: 10.1016/j.bmcl.2011.01.071. Epub 2011 Jan 22.
- 3,7-O-Diacetylpinobanksin
Catalog No.:BCN5849
CAS No.:103553-98-6
- Huperzine B
Catalog No.:BCN1059
CAS No.:103548-82-9
- Apiosylskimmin
Catalog No.:BCN2455
CAS No.:103529-94-8
- AZD4547
Catalog No.:BCC3711
CAS No.:1035270-39-3
- AZ505 ditrifluoroacetate
Catalog No.:BCC4265
CAS No.:1035227-44-1
- AZ505
Catalog No.:BCC4264
CAS No.:1035227-43-0
- Phyllanthin
Catalog No.:BCN5848
CAS No.:10351-88-9
- 9-Dehydroandrostenedione
Catalog No.:BCC8801
CAS No.:1035-69-4
- Fmoc-D-N-Me-Leu-OH
Catalog No.:BCC3346
CAS No.:103478-63-3
- Fmoc-N-Me-Leu-OH
Catalog No.:BCC3345
CAS No.:103478-62-2
- Fmoc-D-N- Me-Val-OH
Catalog No.:BCC3359
CAS No.:103478-58-6
- Diacetylpiptocarphol
Catalog No.:BCN4737
CAS No.:103476-99-9
- Lansoprazole
Catalog No.:BCC1058
CAS No.:103577-45-3
- P005672 hydrochloride
Catalog No.:BCC6406
CAS No.:1035979-44-2
- Isookanin
Catalog No.:BCN6476
CAS No.:1036-49-3
- 5,7-Dimethoxyflavanone
Catalog No.:BCN3569
CAS No.:1036-72-2
- RETRA hydrochloride
Catalog No.:BCC2415
CAS No.:1036069-26-7
- Janolusimide
Catalog No.:BCN1840
CAS No.:103612-45-9
- H-Phe(2-Cl)-OH
Catalog No.:BCC3165
CAS No.:103616-89-3
- (±)-5'-Chloro-5'-deoxy-ENBA
Catalog No.:BCC7716
CAS No.:103626-26-2
- Sumatriptan
Catalog No.:BCC5645
CAS No.:103628-46-2
- Sumatriptan Succinate
Catalog No.:BCC2502
CAS No.:103628-48-4
- Cnidimol A
Catalog No.:BCN7167
CAS No.:103629-80-7
- Catechin 3-rhamnoside
Catalog No.:BCN5850
CAS No.:103630-03-1
Antitumor activity of a potent MEK inhibitor, TAK-733, against colorectal cancer cell lines and patient derived xenografts.[Pubmed:26439693]
Oncotarget. 2015 Oct 27;6(33):34561-72.
BACKGROUND: CRC is a significant cause of cancer mortality, and new therapies are needed for patients with advanced disease. TAK-733 is a highly potent and selective investigational novel MEK allosteric site inhibitor. MATERIALS AND METHODS: In a preclinical study of TAK-733, a panel of CRC cell lines were exposed to varying concentrations of the agent for 72 hours followed by a sulforhodamine B assay. Twenty patient-derived colorectal cancer xenografts were then treated with TAK-733 in vivo. Tumor growth inhibition index (TGII) was assessed to evaluate the sensitivity of the CRC explants to TAK-733 while linear regression was utilized to investigate the predictive effects of genotype on the TGII of explants. RESULTS: Fifty-four CRC cell lines were exposed to TAK-733, while 42 cell lines were deemed sensitive across a broad range of mutations. Eighty-two percent of the cell lines within the sensitive subset were BRAF or KRAS/NRAS mutant, whereas 80% of the cell lines within the sensitive subset were PIK3CA WT. Twenty patient-derived human tumor CRC explants were then treated with TAK-733. In total, 15 primary human tumor explants were found to be sensitive to TAK-733 (TGII 100%). Explants with a BRAF/KRAS/NRAS mutant and PIK3CA wild-type genotype demonstrated increased sensitivity to TAK-733 with a median TGII of -6%. MEK-response gene signatures also correlated with responsiveness to TAK-733 in KRAS-mutant CRC. CONCLUSIONS: The MEK inhibitor TAK-733 demonstrated robust antitumor activity against CRC cell lines and patient-derived tumor explants. While the preclinical activity observed in this study was considerable, single-agent efficacy in the clinic has been limited in CRC, supporting the use of these models in an iterative manner to elucidate resistance mechanisms that can guide rational combination strategies.
A phase I dose-escalation study of TAK-733, an investigational oral MEK inhibitor, in patients with advanced solid tumors.[Pubmed:27650277]
Invest New Drugs. 2017 Feb;35(1):47-58.
Purpose TAK-733, an investigational, selective, allosteric MEK1/2 inhibitor, has demonstrated antitumor effects against multiple cancer cell lines and xenograft models. This first-in-human study investigated TAK-733 in patients with solid tumors. Methods Patients received oral TAK-733 once daily on days 1-21 in 28-day treatment cycles. Adverse events (AEs) were graded using the Common Terminology Criteria for AEs version 3.0. Response was assessed using RECIST v1.1. Blood samples for TAK-733 pharmacokinetics and pharmacodynamics (inhibition of ERK phosphorylation) were collected during cycle 1. Results Fifty-one patients received TAK-733 0.2-22 mg. Primary diagnoses included uveal melanoma (24 %), colon cancer (22 %), and cutaneous melanoma (10 %). Four patients had dose-limiting toxicities of dermatitis acneiform, plus fatigue and pustular rash in one patient, and stomatitis in one patient. The maximum tolerated dose was 16 mg. Common drug-related AEs included dermatitis acneiform (51 %), diarrhea (29 %), and increased blood creatine phosphokinase (20 %); grade >/= 3 AEs were reported in 27 (53 %) patients. Median Tmax was 3 h; systemic exposure increased less than dose-proportionally over the dose range 0.2-22 mg. On day 21 maximum inhibition of ERK phosphorylation in peripheral blood mononuclear cells of 46-97 % was seen in patients receiving TAK-733 >/= 8.4 mg. Among 41 response-evaluable patients, 2 (5 %) patients with cutaneous melanoma (one with BRAF L597R mutant melanoma) had partial responses. Conclusions TAK-733 had a generally manageable toxicity profile up to the maximum tolerated dose, and showed the anticipated pharmacodynamic effect of sustained inhibition of ERK phosphorylation. Limited antitumor activity was demonstrated. Further investigation is not currently planned.
Evaluation of the therapeutic efficacy of a MEK inhibitor (TAK-733) using (1)(8)F-fluorodeoxyglucose-positron emission tomography in the human lung xenograft model A549.[Pubmed:26014721]
Ann Nucl Med. 2015 Aug;29(7):613-20.
OBJECTIVE: The aim of this study was to evaluate the potential of (18)F-fluorodeoxyglucose-positron emission tomography ((18)F-FDG-PET) for monitoring the therapeutic efficacy of TAK-733, an inhibitor of mitogen-activated protein kinase kinase, in nude rats bearing A549 (human lung carcinoma) xenografts. METHODS: TAK-733 was administered orally by gavage to nude xenograft rats for 2 weeks, at dosage levels of 0 (0.5% w/v methylcellulose solution), 1, 3, and 10 mg/kg/day (n = 8/dose). Tumor size was measured before treatment (day 0), and on days 1, 3, 7, 9, 11, and 14. PET scans were performed pretreatment (day 0), and on days 2, 4, 7, 10, and 14. Tracer accumulations in tumor tissue were quantified as the mean standard uptake value (SUVmean). RESULTS: No deaths or treatment-related body weight losses occurred during the study period. TAK-733 showed dose-dependent inhibition of tumor growth and (18)F-FDG uptake in tumor tissue. At a dosage of 10 mg/kg, TAK-733 treatment produced a statistically significant reduction in tumor weight from day 11 compared with the vehicle group (P < 0.05). Tumor growth was inhibited in the 10 mg/kg group with a treated/control value of 31% on day 14. The SUVmean on day 2 in this dosage group was statistically lower than that observed on day 0, and that seen in the vehicle group on day 2 (P < 0.05 for both comparisons). Furthermore, this reduction in SUVmean at 10 mg/kg was maintained over time. In the two lower dosage groups (1 and 3 mg/kg), SUVmean gradually increased over time. CONCLUSIONS: (18)F-FDG-PET enabled early determination of late anti-tumor activity in response to TAK-733 treatment.


