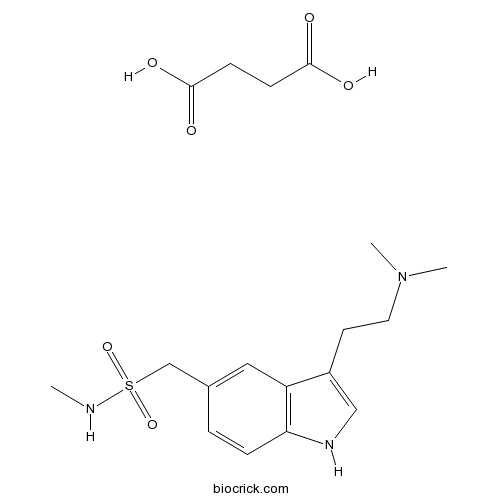
Sumatriptan Succinate5-HT1 receptor agonist CAS# 103628-48-4 |
- Sulfo-NHS-Biotin
Catalog No.:BCC3576
CAS No.:119616-38-5
- Sulfo-NHS-LC-Biotin
Catalog No.:BCC3578
CAS No.:127062-22-0
- NHS-Biotin
Catalog No.:BCC3577
CAS No.:35013-72-0
- Biotin Hydrazide
Catalog No.:BCC3582
CAS No.:66640-86-6
- NHS-LC-Biotin
Catalog No.:BCC3579
CAS No.:72040-63-2
- Iodoacetyl-LC-Biotin
Catalog No.:BCC3584
CAS No.:93285-75-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 103628-48-4 | SDF | Download SDF |
| PubChem ID | 59772 | Appearance | Powder |
| Formula | C18H27N3O6S | M.Wt | 413.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GR 43175 | ||
| Solubility | Soluble to 100 mM in water and to 75 mM in DMSO | ||
| Chemical Name | butanedioic acid;1-[3-[2-(dimethylamino)ethyl]-1H-indol-5-yl]-N-methylmethanesulfonamide | ||
| SMILES | CNS(=O)(=O)CC1=CC2=C(C=C1)NC=C2CCN(C)C.C(CC(=O)O)C(=O)O | ||
| Standard InChIKey | PORMUFZNYQJOEI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H21N3O2S.C4H6O4/c1-15-20(18,19)10-11-4-5-14-13(8-11)12(9-16-14)6-7-17(2)3;5-3(6)1-2-4(7)8/h4-5,8-9,15-16H,6-7,10H2,1-3H3;1-2H2,(H,5,6)(H,7,8) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 5-HT1 receptor agonist (Ki values are 17, 27 and 100 nM at 5-HT1D, 5-HT1B and 5-HT1A receptors respectively). Antimigraine agent. |

Sumatriptan Succinate Dilution Calculator

Sumatriptan Succinate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4184 mL | 12.0922 mL | 24.1844 mL | 48.3688 mL | 60.461 mL |
| 5 mM | 0.4837 mL | 2.4184 mL | 4.8369 mL | 9.6738 mL | 12.0922 mL |
| 10 mM | 0.2418 mL | 1.2092 mL | 2.4184 mL | 4.8369 mL | 6.0461 mL |
| 50 mM | 0.0484 mL | 0.2418 mL | 0.4837 mL | 0.9674 mL | 1.2092 mL |
| 100 mM | 0.0242 mL | 0.1209 mL | 0.2418 mL | 0.4837 mL | 0.6046 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sumatriptan succinate is a selective 5-HT1 receptor agonist with specificity towards 5-HT1D, 5-HT1B and 5-HT1A. In addition, the physicochemical properties of Sumatriptan succinate can be characterized using FT-IR, HPLC, SEM and XRD. It has been reported
- Sumatriptan
Catalog No.:BCC5645
CAS No.:103628-46-2
- (±)-5'-Chloro-5'-deoxy-ENBA
Catalog No.:BCC7716
CAS No.:103626-26-2
- H-Phe(2-Cl)-OH
Catalog No.:BCC3165
CAS No.:103616-89-3
- Janolusimide
Catalog No.:BCN1840
CAS No.:103612-45-9
- RETRA hydrochloride
Catalog No.:BCC2415
CAS No.:1036069-26-7
- 5,7-Dimethoxyflavanone
Catalog No.:BCN3569
CAS No.:1036-72-2
- Isookanin
Catalog No.:BCN6476
CAS No.:1036-49-3
- P005672 hydrochloride
Catalog No.:BCC6406
CAS No.:1035979-44-2
- Lansoprazole
Catalog No.:BCC1058
CAS No.:103577-45-3
- TAK-733
Catalog No.:BCC4587
CAS No.:1035555-63-5
- 3,7-O-Diacetylpinobanksin
Catalog No.:BCN5849
CAS No.:103553-98-6
- Huperzine B
Catalog No.:BCN1059
CAS No.:103548-82-9
- Cnidimol A
Catalog No.:BCN7167
CAS No.:103629-80-7
- Catechin 3-rhamnoside
Catalog No.:BCN5850
CAS No.:103630-03-1
- Ondansetron hydrochloride dihydrate
Catalog No.:BCC4213
CAS No.:103639-04-9
- 2-Carbamoyl-3-hydroxy-1,4-naphthoquinone
Catalog No.:BCC8567
CAS No.:103646-20-4
- Mirificin
Catalog No.:BCN2783
CAS No.:103654-50-8
- Dihydrobonducellin
Catalog No.:BCN3731
CAS No.:103680-87-1
- YK-4-279
Catalog No.:BCC2065
CAS No.:1037184-44-3
- Rehmaglutin D
Catalog No.:BCN5851
CAS No.:103744-84-9
- Fasudil
Catalog No.:BCC5262
CAS No.:103745-39-7
- Shionone
Catalog No.:BCN1274
CAS No.:10376-48-4
- R428
Catalog No.:BCC3692
CAS No.:1037624-75-1
- Gimeracil
Catalog No.:BCC2296
CAS No.:103766-25-2
In-vitro characterization of buccal iontophoresis: the case of sumatriptan succinate.[Pubmed:27113869]
Int J Pharm. 2016 Jun 15;506(1-2):420-8.
Buccal administration of Sumatriptan Succinate might be an interesting alternative to the present administration routes, due to its non-invasiveness and rapid onset of action, but because of its low permeability, a permeation enhancement strategy is required. The aim of this work was then to study, in-vitro, buccal iontophoresis of Sumatriptan Succinate. Permeation experiments were performed in-vitro across pig esophageal epithelium, a recently proposed model of human buccal mucosa, using vertical diffusion cells. The iontophoretic behavior of the tissue was characterized by measuring its isoelectric point (Na(+) transport number and the electroosmotic flow of acetaminophen determination) and by evaluating tissue integrity after current application. The results obtained confirm the usefulness of pig esophageal epithelium as an in-vitro model membrane for buccal drug delivery. The application of iontophoresis increased sumatriptan transport, proportionally to the current density applied, without tissue damage: electrotransport was the predominant mechanism. Integrating the results of the present work with literature data on the transport of other molecules across the buccal mucosa and across the skin, we can draw a general conclusion: the difference in passive transport across buccal mucosa and across the skin is influenced by permeant lipophilicity and by the penetration pathway. Finally, buccal iontophoretic administration of sumatriptan allows to administer 6mg of the drug in 1h, representing a promising alternative to the current administration routes.
A Comparative Study of Orally Delivered PBCA and ApoE Coupled BSA Nanoparticles for Brain Targeting of Sumatriptan Succinate in Therapeutic Management of Migraine.[Pubmed:27003706]
Pharm Res. 2016 Jul;33(7):1682-95.
PURPOSE: The present investigation aimed at brain targeting of Sumatriptan Succinate (SS) for its optimal therapeutic effect in migraine through nanoparticulate drug delivery system using poly (butyl cyanoacrylate) (PBCA) and bovine serum albumin linked with apolipoprotein E3 (BSA-ApoE). METHOD: The study involved formulation optimization of PBCA nanoparticles (NPs) using central composite design for achieving minimum particle size, maximum entrapment efficiency along with sustained drug release. SS incorporated in BSA-ApoE NPs (S-AA-NP) were prepared by desolvation technique and compared with SS loaded polysorbate 80 coated optimized PBCA NPs (FPopt) in terms of their brain uptake potential, upon oral administration in male Wistar rats. The NPs were characterized by FTIR, thermal, powder XRD and TEM analysis. RESULTS: The in vivo studies of FPopt and S-AA-NP on male Wistar rats demonstrated a fairly high brain/plasma drug ratio of 9.45 and 12.67 respectively 2 h post oral drug administration. The behavioural studies on male Swiss albino mice affirmed the enhanced anti-migraine potential of S-AA-NP than FPopt (P < 0.001). CONCLUSION: The results of this work, therefore, indicate that BSA-ApoE NPs are significantly better than polysorbate 80 coated PBCA NPs for brain targeting of SS (P < 0.05) and also offer an improved therapeutic strategy for migraine management.
Permeation of sumatriptan succinate across human skin using multiple types of self-dissolving microneedle arrays fabricated from sodium hyaluronate.[Pubmed:26878569]
J Drug Target. 2016 Sep;24(8):752-8.
Available formulations of Sumatriptan Succinate (SS) have low bioavailability or are associated with site reactions. We developed various types of self-dissolving microneedle arrays (MNs) fabricated from sodium hyaluronate as a new delivery system for SS and evaluated their skin permeation and irritation in terms of clinical application. In vitro permeation studies with human skin, physicochemical properties (needle length, thickness and density), and penetration enhancers (glycerin, sodium dodecyl sulfate and lauric acid diethanolamide) were investigated. SS-loaded high-density MNs of 800 microm in length were the optimal formulation and met clinical therapeutic requirements. Penetration enhancers did not significantly affect permeation of SS from MNs. Optical coherence tomography images demonstrated that SS-loaded high-density MNs (800 microm) uniformly created drug permeation pathways for the delivery of SS into the skin. SS-loaded high-density MNs induced moderate primary skin irritations in rats, but the skin recovered within 72 h of removal of the MNs. These findings suggest that high-density MNs of 800 microm in length are an effective and promising formulation for transdermal delivery of SS. To our knowledge, this is the first report of SS permeation across human skin using self-dissolving MNs.
Comparative Study Between Different Ready-Made Orally Disintegrating Platforms for the Formulation of Sumatriptan Succinate Sublingual Tablets.[Pubmed:27038484]
AAPS PharmSciTech. 2017 Feb;18(2):410-423.
Sumatriptan Succinate (SS) is a selective serotonin receptor agonist used for the treatment of migraine attacks, suffering from extensive first-pass metabolism and low oral bioavailability ( approximately 14%). The aim of this work is to compare the performance of different ready-made co-processed platforms (Pharmaburst(R), Prosolv ODT(R), Starlac(R), Pearlitol Flash(R), or Ludiflash(R)) in the formulation of SS sublingual orodispersible tablets (ODTs) using direct compression technique. The prepared SS ODT formulae were evaluated regarding hardness, friability, simulated wetting time, and in vitro disintegration and dissolution tests. Different mucoadhesive polymers-HPMC K4M, Carbopol(R), chitosan, or Polyox(R)-were tested aiming to increase the residence time in the sublingual area. A pharmacokinetic study on healthy human volunteers was performed, using LC/MS/MS assay, to compare the optimum sublingual formula (Ph25/HPMC) with the conventional oral tablet Imitrex(R). Results showed that tablets prepared using Pharmaburst(R) had significantly (p < 0.05) the lowest simulated wetting and in vitro disintegration times of 17.17 and 23.50 s, respectively, with Q 5 min of 83.62%. HPMC showed a significant (p < 0.05) increase in the residence time from 48.44 to 183.76 s. The relative bioavailability was found to be equal to 132.34% relative to the oral tablet Imitrex(R). In conclusion, Pharmaburst(R) was chosen as the optimum ready-made co-processed platform that can be successfully used in the preparation of SS sublingual tablets for the rapid relief of migraine attacks.
Effect of sumatriptan in different models of pain in rats.[Pubmed:15306203]
Eur J Pharmacol. 2004 Aug 23;497(2):181-6.
The effect of sumatriptan in two standard algesimetric tests and in a model of cephalalgia was evaluated in rats. The pain threshold was measured by the hot-plate and the writhing tests; cephalalgia was produced by injecting bradykinin (10 microg in a volume of 10 microl) into a common carotid artery. Sumatriptan was subcutaneously (s.c.) injected at the doses of 4, 8, 24 or 42 mg/kg; morphine (5 or 10 mg/kg s.c.) and indomethacin (5 or 10 mg/kg s.c) were used as standard analgesic drugs. Sumatriptan had no analgesic activity either in the hot-plate test or in the writhing test. On the other hand, at 24 and 42 mg/kg it dose-dependently reduced the response to the intracarotid injection of bradykinin (vocalization and tachypnea), this effect being prevented by the 5-HT(1B) receptor antagonist, isamoltane. The 5-HT(1D) receptor antagonist BRL15572 prevented the effect of sumatriptan on bradykinin-induced tachypnea, but not the effect of sumatriptan on bradykinin-induced vocalization. These data demonstrate that sumatriptan is significantly effective in a reliable animal model of cephalalgia, while having no systemic analgesic activity.
Sumatriptan in acute migraine: pharmacology and review of world experience.[Pubmed:2177047]
Headache. 1990;30 Suppl 2:554-60.
The introduction of sumatriptan, a novel abortive antimigraine agent, has generated a significant amount of preclinical and clinical interest during the past few years. At the scientific level, sumatriptan is unique in terms of its selective pharmacological properties. The effects of sumatriptan in various experimental paradigms have led to new insights into the pathophysiology of migraine. At the clinical level, sumatriptan appears to be an effective abortive anti-migraine agent with minimal side effects. Its ability to decrease, rather than exacerbate, the nausea and vomiting of migraine appears to be an important advance in the treatment of migraine.
Sumatriptan (GR 43175) interacts selectively with 5-HT1B and 5-HT1D binding sites.[Pubmed:2545459]
Eur J Pharmacol. 1989 Apr 12;163(1):133-6.
The ability of sumatriptan (GR 43175; 3-[2-dimethylamino]ethyl-N-methyl-1H-indole-5 methane sulphonamide) to interact with 13 neurotransmitter receptor sites was determined using radioligand binding techniques. Sumatriptan displayed the highest affinity for 5-HT1D (Ki = 17 nM) and 5-HT1B (Ki = 27 nM) binding sites and was slightly less potent at 5-HT1A binding sites (Ki = 100 nM). By contrast, sumatriptan was essentially inactive (Ki greater than 10,000 nM) at each of the 10 other binding sites analyzed. These data indicate that sumatriptan interacts selectively with 5-HT1B and 5-HT1D sites and suggest that these interactions may be the basis of its apparent efficacy in the acute treatment of migraine.


