Huperzine BCAS# 103548-82-9 |
Quality Control & MSDS
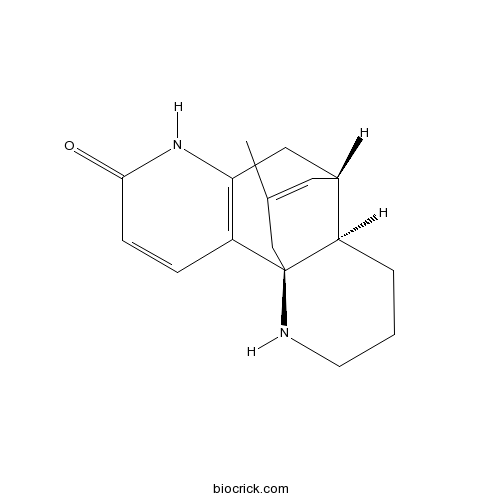
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 103548-82-9 | SDF | Download SDF |
| PubChem ID | 5462442 | Appearance | White powder |
| Formula | C16H20N2O | M.Wt | 256.35 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Fordimine | ||
| Solubility | Soluble in DMSO and methanol; insoluble in water | ||
| SMILES | CC1=CC2CC3=C(C=CC(=O)N3)C4(C1)C2CCCN4 | ||
| Standard InChIKey | YYWGABLTRMRUIT-HWWQOWPSSA-N | ||
| Standard InChI | InChI=1S/C16H20N2O/c1-10-7-11-8-14-13(4-5-15(19)18-14)16(9-10)12(11)3-2-6-17-16/h4-5,7,11-12,17H,2-3,6,8-9H2,1H3,(H,18,19)/t11-,12+,16+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Huperzine B is a efficient inhibitor of human brain AChE, it can enhance ognitive and protect neuro, may be potentially new drug candidates for Alzheimer's disease therapy. |
| Targets | AChR |
| Kinase Assay | Novel 16-substituted bifunctional derivatives of huperzine B: multifunctional cholinesterase inhibitors.[Pubmed: 19578388]Acta Pharmacol Sin. 2009 Aug;30(8):1195-203.To design novel bifunctional derivatives of Huperzine B (HupB) based on the concept of dual binding site of acetylcholinesterase (AChE) and evaluate their pharmacological activities for seeking new drug candidates against Alzheimer's disease (AD). |
| Structure Identification | Bioorg Med Chem. 2007 Feb 1;15(3):1394-408.Study on dual-site inhibitors of acetylcholinesterase: Highly potent derivatives of bis- and bifunctional huperzine B.[Pubmed: 17126020]Natural (-)-Huperzine B (HupB), isolated from Chinese medicinal herb, displayed moderate inhibitory activity of acetylcholinesterase (AChE). |

Huperzine B Dilution Calculator

Huperzine B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9009 mL | 19.5046 mL | 39.0092 mL | 78.0183 mL | 97.5229 mL |
| 5 mM | 0.7802 mL | 3.9009 mL | 7.8018 mL | 15.6037 mL | 19.5046 mL |
| 10 mM | 0.3901 mL | 1.9505 mL | 3.9009 mL | 7.8018 mL | 9.7523 mL |
| 50 mM | 0.078 mL | 0.3901 mL | 0.7802 mL | 1.5604 mL | 1.9505 mL |
| 100 mM | 0.039 mL | 0.195 mL | 0.3901 mL | 0.7802 mL | 0.9752 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Apiosylskimmin
Catalog No.:BCN2455
CAS No.:103529-94-8
- AZD4547
Catalog No.:BCC3711
CAS No.:1035270-39-3
- AZ505 ditrifluoroacetate
Catalog No.:BCC4265
CAS No.:1035227-44-1
- AZ505
Catalog No.:BCC4264
CAS No.:1035227-43-0
- Phyllanthin
Catalog No.:BCN5848
CAS No.:10351-88-9
- 9-Dehydroandrostenedione
Catalog No.:BCC8801
CAS No.:1035-69-4
- Fmoc-D-N-Me-Leu-OH
Catalog No.:BCC3346
CAS No.:103478-63-3
- Fmoc-N-Me-Leu-OH
Catalog No.:BCC3345
CAS No.:103478-62-2
- Fmoc-D-N- Me-Val-OH
Catalog No.:BCC3359
CAS No.:103478-58-6
- Diacetylpiptocarphol
Catalog No.:BCN4737
CAS No.:103476-99-9
- Leupeptin, Microbial
Catalog No.:BCC1217
CAS No.:103476-89-7
- Maprotiline HCl
Catalog No.:BCC4329
CAS No.:10347-81-6
- 3,7-O-Diacetylpinobanksin
Catalog No.:BCN5849
CAS No.:103553-98-6
- TAK-733
Catalog No.:BCC4587
CAS No.:1035555-63-5
- Lansoprazole
Catalog No.:BCC1058
CAS No.:103577-45-3
- P005672 hydrochloride
Catalog No.:BCC6406
CAS No.:1035979-44-2
- Isookanin
Catalog No.:BCN6476
CAS No.:1036-49-3
- 5,7-Dimethoxyflavanone
Catalog No.:BCN3569
CAS No.:1036-72-2
- RETRA hydrochloride
Catalog No.:BCC2415
CAS No.:1036069-26-7
- Janolusimide
Catalog No.:BCN1840
CAS No.:103612-45-9
- H-Phe(2-Cl)-OH
Catalog No.:BCC3165
CAS No.:103616-89-3
- (±)-5'-Chloro-5'-deoxy-ENBA
Catalog No.:BCC7716
CAS No.:103626-26-2
- Sumatriptan
Catalog No.:BCC5645
CAS No.:103628-46-2
- Sumatriptan Succinate
Catalog No.:BCC2502
CAS No.:103628-48-4
Study on dual-site inhibitors of acetylcholinesterase: Highly potent derivatives of bis- and bifunctional huperzine B.[Pubmed:17126020]
Bioorg Med Chem. 2007 Feb 1;15(3):1394-408.
Natural (-)-Huperzine B (HupB), isolated from Chinese medicinal herb, displayed moderate inhibitory activity of acetylcholinesterase (AChE). Based on the active dual-site of AChE, a series of novel derivatives of bis- and bifunctional HupB were designed and synthesized. The AChE inhibition potency of most derivatives of HupB was enhanced about 2-3 orders of magnitude as compared with the parental HupB. Among bis-HupB derivatives, 12h exhibited the most potent in the AChE inhibition and has been evaluated for its pharmacological actions in vivo on ChE inhibition, cognitive enhancement, and neuroprotection. The docking study on the bis-HupB derivatives 12 series with TcAChE has demonstrated that the ligands bound to the dual-site of the enzyme in different level.
Novel 16-substituted bifunctional derivatives of huperzine B: multifunctional cholinesterase inhibitors.[Pubmed:19578388]
Acta Pharmacol Sin. 2009 Aug;30(8):1195-203.
AIM: To design novel bifunctional derivatives of Huperzine B (HupB) based on the concept of dual binding site of acetylcholinesterase (AChE) and evaluate their pharmacological activities for seeking new drug candidates against Alzheimer's disease (AD). METHODS: Novel 16-substituted bifunctional derivatives of HupB were synthesized through chemical reactions. The inhibitory activities of the derivatives toward AChE and butyrylcholinesterase (BuChE) were determined in vitro by modified Ellman's method. Cell viability was quantified by the reduction of MTT. RESULTS: A new preparative method was developed for the generation of 16-substituted derivatives of HupB, and pharmacological trials indicated that the derivatives were multifunctional cholinesterase inhibitors targeting both AChE and BuChE. Among the derivatives tested, 9c, 9e, 9f, and 9i were 480 to 1360 times more potent as AChE inhibitors and 370 to 1560 times more potent as BuChE inhibitors than the parent HupB. Further preliminary pharmacological trials of derivatives 9c and 9i were performed, including examining the mechanism of AChE inhibition, the substrate kinetics of the enzyme inhibition, and protection against hydrogen peroxide (H2O2)-induced cytotoxicity in PC12 cells. CONCLUSION: Preliminary pharmacological evaluation indicated that 16-substituted derivatives of HupB, particularly 9c and 9i, would be potentially valuable new drug candidates for AD therapy, and further exploration is needed to evaluate their pharmacological and clinical efficacies.
Molecular interaction of human brain acetylcholinesterase with a natural inhibitor huperzine-B: an enzoinformatics approach.[Pubmed:24059299]
CNS Neurol Disord Drug Targets. 2014 Apr;13(3):487-90.
The present study emphasizes the molecular interactions between human brain acetylcholinesterase (AChE) and the natural ligand Huperzine-B and its comparison to 'AChE-Tolserine interactions'. Docking between Huperzine-B and AChE was performed using 'Autodock4.2'. Hydrophobic interactions and hydrogen bonds both play an equally important role in the correct positioning of Huperzine-B within the 'catalytic site' of AChE to permit docking. However, docking of Tolserine to AChE is largely dominated by hydrophobic interactions. Such information may aid in the design of versatile AChE-inhibitors, and is expected to aid in safe clinical use of Huperzine-B. Scope still remains in the determination of the three-dimensional structure of AChE-Huperzine-B complex by X-ray crystallography to validate the described data. Furthermore, this study confirms that Huperzine-B is a more efficient inhibitor of human brain AChE compared to tolserine with reference to Ki and DeltaG values.
Synthesis and acetylcholinesterase inhibition of derivatives of huperzine B.[Pubmed:15664805]
Bioorg Med Chem Lett. 2005 Feb 1;15(3):523-6.
By targeting dual active sites of AChE, a number of new derivatives of HupB have been synthesized and tested as acetylcholinesterase inhibitors. The most potent compound, bis-HupB 5b is 72-fold more potent in AChE inhibition and 79-fold more selective for AChE versus BChE than HupB.


