Cyclo(Tyr-Leu)CAS# 82863-65-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 82863-65-8 | SDF | Download SDF |
| PubChem ID | 572422 | Appearance | Powder |
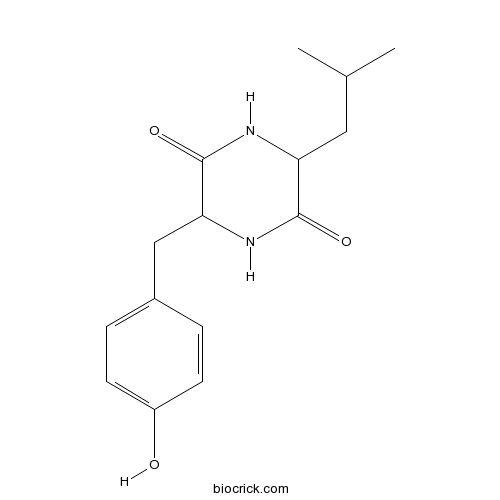
| Formula | C15H20N2O3 | M.Wt | 276.33 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-[(4-hydroxyphenyl)methyl]-6-(2-methylpropyl)piperazine-2,5-dione | ||
| SMILES | CC(C)CC1C(=O)NC(C(=O)N1)CC2=CC=C(C=C2)O | ||
| Standard InChIKey | GENSLUDVKWKQMX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H20N2O3/c1-9(2)7-12-14(19)17-13(15(20)16-12)8-10-3-5-11(18)6-4-10/h3-6,9,12-13,18H,7-8H2,1-2H3,(H,16,20)(H,17,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cyclo(Tyr-Leu) shows cytotoxicity, antifungal,and anticoagulant activities. |
| Targets | Antifection |
| In vitro | Isolation and antifungal properties of cyclo(D-Tyr-L-Leu) diketopiperazine isolated from Bacillus sp. associated with rhabditid entomopathogenic nematode.[Reference: WebLink]Natural Product Research 2013, 27(23):2168-217Bacillus sp. associated with an entomopathogenic nematode is shown to produce diketopiperazine (DKP) that showed stronger antifungal activity against Colletotrichum gloeosporioides [minimum inhibitory concentration (MIC): 8 μg mL(- 1)] than commercial fungicide oligochitosan (MIC: 125 μg mL(- 1)). The antitumor active component from marine derived actinomycete S1001[Reference: WebLink]Chinese Journal of Antibiotics,2006, 31(1):36-38,62.To explore the antitumor active component in fermentation of a marine derived actinomycete S1001,8 compounds were isolated and purified by using solvent extraction,silica gel column and preparative HPLC. Anticoagulant activity of cyclic dipeptides from Sparganii Rhizome[Reference: WebLink]Chinese Traditional Patent Medicine, 2015,37 (1) : 34-9.To study the in vitro anticoagulant activity of cyclodipeptide Cyclo(Tyr-Leu),cyclo-( PhePhe) and cyclo-( Phe-Tyr)) isolated from Sparganium stoloniferum Buch.-Ham. |

Cyclo(Tyr-Leu) Dilution Calculator

Cyclo(Tyr-Leu) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6189 mL | 18.0943 mL | 36.1886 mL | 72.3772 mL | 90.4715 mL |
| 5 mM | 0.7238 mL | 3.6189 mL | 7.2377 mL | 14.4754 mL | 18.0943 mL |
| 10 mM | 0.3619 mL | 1.8094 mL | 3.6189 mL | 7.2377 mL | 9.0472 mL |
| 50 mM | 0.0724 mL | 0.3619 mL | 0.7238 mL | 1.4475 mL | 1.8094 mL |
| 100 mM | 0.0362 mL | 0.1809 mL | 0.3619 mL | 0.7238 mL | 0.9047 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Echinacoside
Catalog No.:BCN4953
CAS No.:82854-37-3
- Pingpeimine B
Catalog No.:BCN8408
CAS No.:82851-52-3
- Pingpeimine A
Catalog No.:BCN8407
CAS No.:82841-67-6
- Perindopril
Catalog No.:BCC4223
CAS No.:82834-16-0
- cGMP Dependent Kinase Inhibitor Peptide
Catalog No.:BCC8084
CAS No.:82801-73-8
- H-D-HoPhe-OH
Catalog No.:BCC3239
CAS No.:82795-51-5
- Nefazodone hydrochloride
Catalog No.:BCC7479
CAS No.:82752-99-6
- DCPIB
Catalog No.:BCC7105
CAS No.:82749-70-0
- 6,7-Dihydroxy-4-(Trifluoromethyl)Coumarin
Catalog No.:BCC8286
CAS No.:82747-36-2
- Perforatumone
Catalog No.:BCN4370
CAS No.:827319-50-6
- Danusertib (PHA-739358)
Catalog No.:BCC2172
CAS No.:827318-97-8
- Palbociclib (PD0332991) Isethionate
Catalog No.:BCC3698
CAS No.:827022-33-3
- 2,3-Dihydroisoginkgetin
Catalog No.:BCN4035
CAS No.:828923-27-9
- (R)-(+)-Etomoxir sodium salt
Catalog No.:BCC7946
CAS No.:828934-41-4
- 3-Oxo-24,25,26,27-tetranortirucall-7-en-23,21-olide
Catalog No.:BCN1338
CAS No.:828935-47-3
- Fmoc-Osu
Catalog No.:BCC2804
CAS No.:82911-69-1
- 7,4'-Dihydroxy-8-methylflavan
Catalog No.:BCN6841
CAS No.:82925-55-1
- CI 898 trihydrochloride
Catalog No.:BCC7248
CAS No.:82952-64-5
- Nafamostat Mesylate(FUT-175)
Catalog No.:BCC1228
CAS No.:82956-11-4
- 11-O-Galloylbergenin
Catalog No.:BCN6637
CAS No.:82958-44-9
- 4-O-Galloylbergenin
Catalog No.:BCN6643
CAS No.:82958-45-0
- Tolrestat
Catalog No.:BCC4084
CAS No.:82964-04-3
- 4-Aminoantipyrine
Catalog No.:BCC8683
CAS No.:83-07-8
- Phenindione
Catalog No.:BCC4699
CAS No.:83-12-5
Protective effects of diketopiperazines from Moslae Herba against influenza A virus-induced pulmonary inflammation via inhibition of viral replication and platelets aggregation.[Pubmed:29309861]
J Ethnopharmacol. 2018 Apr 6;215:156-166.
ETHNOPHARMACOLOGICAL RELEVANCE: Moslae Herba (MH) is broadly used as an antiviral, antipyretic and anticoagulant drug which effectively treats respiratory diseases including cough, asthma, throat, cold and flu. AIM OF THIS STUDY: The excessive inflammation of the lungs is the hallmark of severe influenza A virus (IAV) infection, while platelet aggregation and its subsequent microvascular thrombosis can exacerbate IAV-induced lung injury. Thus, inhibition of platelet aggregation can be a potential target for IAV treatment. Previous studies focus on the flavonoids from MH and their anti-inflammatory activities, but the anticoagulant compounds and potential molecular mechanism of MH remains unclear. This study was to isolate and characterize diketopiperazines (DKPs) from MH and to explore the underlying anticoagulant mechanism on IAV infection models. MATERIALS AND METHODS: EtOAc sub-extract separated from MH ethanolic extract was subjected to fractionation through column chromatography. The chemical structures of pure compounds were characterized by the spectral analysis. Antiviral activities of DKPs were assayed in IAV-infected Madin-Darby canine kidney (MDCK) cells and mice. Anticoagulant effects of DKPs were investigated on adenosine 5'-diphosphate (ADP)-induced acute pulmonary embolism and IAV-induced lung injury in vivo, as well as the inhibition on platelet activating factor (PAF), arachidonic acid (AA) and ADP-induced platelet aggregation in vitro. The serum levels of thromboxane B2 (TXB2) and 6-keto-PGF1alpha were detected by ELISA. The expressions of key proteins in CD41-mediated PI3K/AKT pathways were determined by western blotting analysis. RESULTS: Six DKPs were, for the first time, isolated from MH and identified as Cyclo(Tyr-Leu) (1), cyclo(Phe-Phe) (2), cyclo(Phe-Tyr) (3), cyclo(Ala-Ile) (4), cyclo(Ala-Leu) (5) and Bz-Phe-Phe-OMe (6). Among these DKPs, cyclo(Ala-Ile) and Bz-Phe-Phe-OMe possessed low cytotoxicities and significant inhibition against cytopathic effects induced by IAV (H1N1 and H3N2) replication in MDCK cells. Furthermore, cyclo(Ala-Ile) and Bz-Phe-Phe-OMe significantly alleviated IAV-induced platelet activation and lung inflammation in mice. They could reduce the expression of CD41 and the phosphorylation of PI3K and AKT in PLTs of IAV-infected mice. CONCLUSION: These results suggested that cyclo(Ala-Ile) and Bz-Phe-Phe-OMe isolated from MH have antiviral and anticoagulant effects against IAV-induced PLT aggregation and lung inflammation via regulating CD41/PI3K/AKT pathway, and could be used as the potential agents for IAV treatment.
Isolation and antifungal properties of cyclo(D-Tyr-L-Leu) diketopiperazine isolated from Bacillus sp. associated with rhabditid entomopathogenic nematode.[Pubmed:23672207]
Nat Prod Res. 2013;27(23):2168-72.
Bacillus sp. associated with an entomopathogenic nematode is shown to produce diketopiperazine (DKP) that showed stronger antifungal activity against Colletotrichum gloeosporioides [minimum inhibitory concentration (MIC): 8 mug mL(- 1)] than commercial fungicide oligochitosan (MIC: 125 mug mL(- 1)). DKP identified as cyclo(D-Tyr-L-Leu) was isolated for the first time from a natural source with a d-tyrosine residue. This report also demonstrates for the first time an antifungal property exploration of Cyclo(Tyr-Leu) class of dipeptides. The structural elucidation was carried out using 1D, 2D NMR methods and HPLC.


