PhenindioneCAS# 83-12-5 |
- Metformin HCl
Catalog No.:BCC4799
CAS No.:1115-70-4
- Lenalidomide (CC-5013)
Catalog No.:BCC2245
CAS No.:191732-72-6
- Gliclazide
Catalog No.:BCC5002
CAS No.:21187-98-4
- Geniposide
Catalog No.:BCN5104
CAS No.:24512-63-8
- MEK inhibitor
Catalog No.:BCC1738
CAS No.:334951-92-7
- Imeglimin hydrochloride
Catalog No.:BCC4085
CAS No.:775351-61-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 83-12-5 | SDF | Download SDF |
| PubChem ID | 4760 | Appearance | Powder |
| Formula | C15H10O2 | M.Wt | 222.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Rectadione | ||
| Solubility | DMSO : ≥ 100 mg/mL (449.96 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
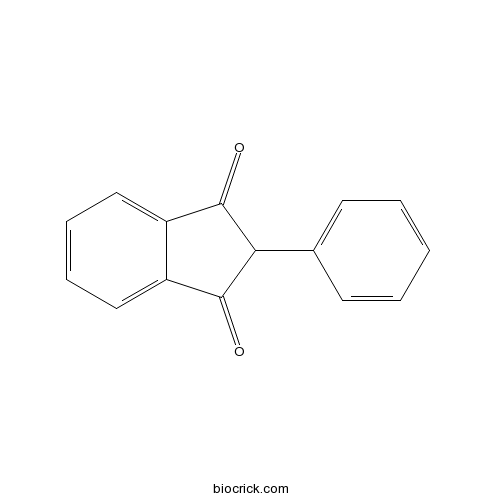
| Chemical Name | 2-phenylindene-1,3-dione | ||
| SMILES | C1=CC=C(C=C1)C2C(=O)C3=CC=CC=C3C2=O | ||
| Standard InChIKey | NFBAXHOPROOJAW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H10O2/c16-14-11-8-4-5-9-12(11)15(17)13(14)10-6-2-1-3-7-10/h1-9,13H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Phenindione is an anticoagulant which functions as a Vitamin K antagonist.
Target: Others
Phenindione(Rectadione) is an anticoagulant which functions as a Vitamin K antagonist. A lymphocyte transformation test showed proliferation of T-cells from the hypersensitive patient, but not from four controls on exposure to phenindione in vitro. Drug-specific T-cell clones were generated and characterized in terms of their phenotype, functionality, and mechanism of antigen presentation. Forty-three human leukocyte antigen class II restricted CD4+ αβ T-cell clones were identified. T-cell activation resulted in the secretion of interferon-γ and interleukin-5 [1]. References: | |||||

Phenindione Dilution Calculator

Phenindione Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4996 mL | 22.4982 mL | 44.9964 mL | 89.9928 mL | 112.491 mL |
| 5 mM | 0.8999 mL | 4.4996 mL | 8.9993 mL | 17.9986 mL | 22.4982 mL |
| 10 mM | 0.45 mL | 2.2498 mL | 4.4996 mL | 8.9993 mL | 11.2491 mL |
| 50 mM | 0.09 mL | 0.45 mL | 0.8999 mL | 1.7999 mL | 2.2498 mL |
| 100 mM | 0.045 mL | 0.225 mL | 0.45 mL | 0.8999 mL | 1.1249 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Phenindione
- 4-Aminoantipyrine
Catalog No.:BCC8683
CAS No.:83-07-8
- Tolrestat
Catalog No.:BCC4084
CAS No.:82964-04-3
- 4-O-Galloylbergenin
Catalog No.:BCN6643
CAS No.:82958-45-0
- 11-O-Galloylbergenin
Catalog No.:BCN6637
CAS No.:82958-44-9
- Nafamostat Mesylate(FUT-175)
Catalog No.:BCC1228
CAS No.:82956-11-4
- CI 898 trihydrochloride
Catalog No.:BCC7248
CAS No.:82952-64-5
- 7,4'-Dihydroxy-8-methylflavan
Catalog No.:BCN6841
CAS No.:82925-55-1
- Fmoc-Osu
Catalog No.:BCC2804
CAS No.:82911-69-1
- 3-Oxo-24,25,26,27-tetranortirucall-7-en-23,21-olide
Catalog No.:BCN1338
CAS No.:828935-47-3
- (R)-(+)-Etomoxir sodium salt
Catalog No.:BCC7946
CAS No.:828934-41-4
- 2,3-Dihydroisoginkgetin
Catalog No.:BCN4035
CAS No.:828923-27-9
- Cyclo(Tyr-Leu)
Catalog No.:BCN2432
CAS No.:82863-65-8
- 1-Indanone
Catalog No.:BCN2245
CAS No.:83-33-0
- Methylprednisolone
Catalog No.:BCC2256
CAS No.:83-43-2
- Deoxycholic acid
Catalog No.:BCN1288
CAS No.:83-44-3
- Beta-Sitosterol
Catalog No.:BCN1015
CAS No.:83-46-5
- Stigmasterol
Catalog No.:BCN4376
CAS No.:83-48-7
- Hyodeoxycholic acid
Catalog No.:BCN1287
CAS No.:83-49-8
- 5-Amino-1-naphthol
Catalog No.:BCC8729
CAS No.:83-55-6
- Theobromine
Catalog No.:BCN1227
CAS No.:83-67-0
- 2-Hydroxy-1,4-naphoquinone
Catalog No.:BCN8398
CAS No.:83-72-7
- Ibogaine
Catalog No.:BCN4378
CAS No.:83-74-9
- Rotenone
Catalog No.:BCN5412
CAS No.:83-79-4
- Phytic acid
Catalog No.:BCN1282
CAS No.:83-86-3
Keto-enol tautomerism of phenindione and its derivatives: an NMR and density functional theory (DFT) reinvestigation.[Pubmed:25629727]
J Phys Chem A. 2015 Feb 26;119(8):1404-14.
Keto-enol tautomerism of Phenindione (2-phenyl-1,3-indandione) and of its 4-phenyl-substituted derivatives was reinvestigated by NMR, supported by density functional theory (DFT) quantum-mechanical calculations. The calculated data quantitatively confirmed the stabilization in DMSO solution of the enol form by a strong hydrogen bond. The symmetry of the NMR spectra of the enol forms was explained by a fast proton transfer between carbonyl oxygen atoms, which is facilitated by the formation of a strong ionic complex of the enol form and an anion. It was shown that keto-enol tautomerization also proceeds with the participation of a similar complex between an anion and the diketo form of 2-phenyl-1,3-indandione.
Characterization of the T-cell response in a patient with phenindione hypersensitivity.[Pubmed:15743920]
J Pharmacol Exp Ther. 2005 Jun;313(3):1058-65.
The oral anticoagulant Phenindione [2-phenyl-1H-indene-1,3(2H)-dione] is associated with hypersensitivity reactions in 1.5 to 3% of patients, the pathogenesis of which is unclear. We describe a patient who developed a severe hypersensitivity reaction that involved both the skin and lungs. A lymphocyte transformation test showed proliferation of T-cells from the hypersensitive patient, but not from four controls on exposure to Phenindione in vitro. Drug-specific T-cell clones were generated and characterized in terms of their phenotype, functionality, and mechanism of antigen presentation. Forty-three human leukocyte antigen class II restricted CD4(+) alphabeta T-cell clones were identified. T-cell activation resulted in the secretion of interferon-gamma and interleukin-5. Five of seven clones proliferated with Phenindione alone, whereas two clones also proliferated with 2-phenylindene. Certain T-cell clones were also stimulated by R- and S-warfarin; computer modeling revealed that warfarin can adopt a Phenindione-like structure. Phenindione was presented to T-cells via two pathways: first, bound directly to major histocompatibility complex and second, bound to a processed peptide. Our data show that CD4(+) T-cells are involved in the pathophysiology of Phenindione hypersensitivity. There may be cross-sensitivity with warfarin in some Phenindione hypersensitive patients.
Phenindione interference in enzymatic creatinine assay - A case report.[Pubmed:24361058]
Clin Nephrol. 2015 Feb;83(2):121-3.
Enzymatic creatinine assays are considered superior to Jaffe assays due to greater analytical specificity. We report a case of Phenindione interference with an enzymatic assay resulting in significant misclassification in a patient with chronic kidney disease (CKD). Analysis of creatinine values of a further 36 patients who were treated with Phenindione showed significant negative interference of Phenindione with the Roche enzymatic creatinine assay.


