1-IndanoneCAS# 83-33-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 83-33-0 | SDF | Download SDF |
| PubChem ID | 6735 | Appearance | Cryst. |
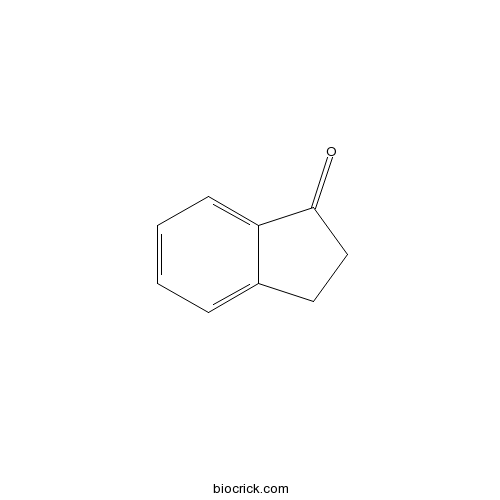
| Formula | C9H8O | M.Wt | 132.16 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2,3-dihydroinden-1-one | ||
| SMILES | C1CC(=O)C2=CC=CC=C21 | ||
| Standard InChIKey | QNXSIUBBGPHDDE-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1-Indanone thiosemicarbazones coordinated to palladium(II) is more cytotoxic than those complexed with platinum(II); although platinum(II) is more selective for leukemic cells and has potential to treat hematological malignancies. |
| In vitro | Antiviral activity against the hepatitis C virus (HCV) of 1-indanone thiosemicarbazones and their inclusion complexes with hydroxypropyl-β-cyclodextrin.[Pubmed: 22885176 ]Eur J Pharm Sci. 2012 Oct 9;47(3):596-603.The hepatitis C virus (HCV) is a major cause of acute and chronic hepatitis in humans. Approximately 5% of the infected people die from cirrhosis or hepatocellular carcinoma. The current standard therapy comprises a combination of pegylated-interferon alpha and ribavirin. Due to the relatively low effectiveness, the prohibitive costs and the extensive side effects of the treatment, an intense research for new direct-acting anti-HCV agents is taking place. Furthermore, NS3 protease inhibitors recently introduced into the market are not effective against all HCV subgenotypes. Thiosemicarbazones (TSCs) have shown antiviral activity against a wide range of DNA and RNA viruses. However, their extremely low aqueous solubility and high self-aggregation tendency often preclude their reliable biological evaluation in vitro. Oxidative potential of some endophytic fungi using 1-indanone as a substrate.[Pubmed: 22573162]J Microbiol Biotechnol. 2012 Jun;22(6):832-7.
|
| Structure Identification | ChemMedChem. 2011 Aug 1;6(8):1485-94.Synthesis, structural characterization, and pro-apoptotic activity of 1-indanone thiosemicarbazone platinum(II) and palladium(II) complexes: potential as antileukemic agents.[Pubmed: 21608131]In the search for alternative chemotherapeutic strategies against leukemia, various 1-Indanone thiosemicarbazones, as well as eight novel platinum(II) and palladium(II) complexes, with the formula [MCl₂(HL)] and [M(HL)(L)]Cl, derived from two 1-Indanone thiosemicarbazones were synthesized and tested for antiproliferative activity against the human leukemia U937 cell line. |

1-Indanone Dilution Calculator

1-Indanone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.5666 mL | 37.8329 mL | 75.6659 mL | 151.3317 mL | 189.1646 mL |
| 5 mM | 1.5133 mL | 7.5666 mL | 15.1332 mL | 30.2663 mL | 37.8329 mL |
| 10 mM | 0.7567 mL | 3.7833 mL | 7.5666 mL | 15.1332 mL | 18.9165 mL |
| 50 mM | 0.1513 mL | 0.7567 mL | 1.5133 mL | 3.0266 mL | 3.7833 mL |
| 100 mM | 0.0757 mL | 0.3783 mL | 0.7567 mL | 1.5133 mL | 1.8916 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Phenindione
Catalog No.:BCC4699
CAS No.:83-12-5
- 4-Aminoantipyrine
Catalog No.:BCC8683
CAS No.:83-07-8
- Tolrestat
Catalog No.:BCC4084
CAS No.:82964-04-3
- 4-O-Galloylbergenin
Catalog No.:BCN6643
CAS No.:82958-45-0
- 11-O-Galloylbergenin
Catalog No.:BCN6637
CAS No.:82958-44-9
- Nafamostat Mesylate(FUT-175)
Catalog No.:BCC1228
CAS No.:82956-11-4
- CI 898 trihydrochloride
Catalog No.:BCC7248
CAS No.:82952-64-5
- 7,4'-Dihydroxy-8-methylflavan
Catalog No.:BCN6841
CAS No.:82925-55-1
- Fmoc-Osu
Catalog No.:BCC2804
CAS No.:82911-69-1
- 3-Oxo-24,25,26,27-tetranortirucall-7-en-23,21-olide
Catalog No.:BCN1338
CAS No.:828935-47-3
- (R)-(+)-Etomoxir sodium salt
Catalog No.:BCC7946
CAS No.:828934-41-4
- 2,3-Dihydroisoginkgetin
Catalog No.:BCN4035
CAS No.:828923-27-9
- Methylprednisolone
Catalog No.:BCC2256
CAS No.:83-43-2
- Deoxycholic acid
Catalog No.:BCN1288
CAS No.:83-44-3
- Beta-Sitosterol
Catalog No.:BCN1015
CAS No.:83-46-5
- Stigmasterol
Catalog No.:BCN4376
CAS No.:83-48-7
- Hyodeoxycholic acid
Catalog No.:BCN1287
CAS No.:83-49-8
- 5-Amino-1-naphthol
Catalog No.:BCC8729
CAS No.:83-55-6
- Theobromine
Catalog No.:BCN1227
CAS No.:83-67-0
- 2-Hydroxy-1,4-naphoquinone
Catalog No.:BCN8398
CAS No.:83-72-7
- Ibogaine
Catalog No.:BCN4378
CAS No.:83-74-9
- Rotenone
Catalog No.:BCN5412
CAS No.:83-79-4
- Phytic acid
Catalog No.:BCN1282
CAS No.:83-86-3
- Riboflavine
Catalog No.:BCN2224
CAS No.:83-88-5
Antiviral activity against the hepatitis C virus (HCV) of 1-indanone thiosemicarbazones and their inclusion complexes with hydroxypropyl-beta-cyclodextrin.[Pubmed:22885176]
Eur J Pharm Sci. 2012 Oct 9;47(3):596-603.
The hepatitis C virus (HCV) is a major cause of acute and chronic hepatitis in humans. Approximately 5% of the infected people die from cirrhosis or hepatocellular carcinoma. The current standard therapy comprises a combination of pegylated-interferon alpha and ribavirin. Due to the relatively low effectiveness, the prohibitive costs and the extensive side effects of the treatment, an intense research for new direct-acting anti-HCV agents is taking place. Furthermore, NS3 protease inhibitors recently introduced into the market are not effective against all HCV subgenotypes. Thiosemicarbazones (TSCs) have shown antiviral activity against a wide range of DNA and RNA viruses. However, their extremely low aqueous solubility and high self-aggregation tendency often preclude their reliable biological evaluation in vitro. In this work, we investigated and compared for the first time the anti-HCV activity of two 1-Indanone TSCs, namely 5,6-dimethoxy-1-Indanone TSC and 5,6-dimethoxy-1-Indanone N4-allyl TSC, and their inclusion complexes with hydroxypropyl-beta-cyclodextrin (HPbeta-CD) in Huh-7.5 cells containing the full-length and the subgenomic subgenotype 1b HCV replicon system. Studies of physical stability in culture medium showed that free TSCs precipitated rapidly and formed submicron aggregates. Conversely, TSC complexation with HPbeta-CD led to more stable systems with minimal size growth and drug concentration loss. More importantly, both TSCs and their inclusion complexes displayed a potent suppression of the HCV replication in both cell lines with no cytotoxic effects. The mechanism likely involves the inhibition of non-structural proteins of the virus. In addition, findings suggested that the cyclodextrin released the drug to the culture medium over time. This platform could be exploited for the study of the drug toxicity and pharmacokinetics animal models.
Oxidative potential of some endophytic fungi using 1-indanone as a substrate.[Pubmed:22573162]
J Microbiol Biotechnol. 2012 Jun;22(6):832-7.
The oxidative potential of the fungus Penicillium brasilianum, a strain isolated as an endophyte from a Meliaceae plant (Melia azedarach), was investigated using 1-Indanone as a substrate to track the production of monooxygenases. The fungus produced the dihydrocoumarin from 1-Indanone with the classical Baeyer-Villiger reaction regiochemistry, and (-)-(R)-3-hydroxy-1-Indanone with 78% ee. Minor compounds resulting from lipase and SAM activities were also detected. The biotransformation procedures were also applied to a collection of Penicillium and Aspergillus fungi obtained from M. azedarach and Murraya paniculata. The results showed that Baeyer-Villiger were mostly active in fungi isolated from M. azedarach. Almost all of the fungi tested produced 3-hydroxy-1-Indanone..
Synthesis, structural characterization, and pro-apoptotic activity of 1-indanone thiosemicarbazone platinum(II) and palladium(II) complexes: potential as antileukemic agents.[Pubmed:21608131]
ChemMedChem. 2011 Aug 1;6(8):1485-94.
In the search for alternative chemotherapeutic strategies against leukemia, various 1-Indanone thiosemicarbazones, as well as eight novel platinum(II) and palladium(II) complexes, with the formula [MCl(2)(HL)] and [M(HL)(L)]Cl, derived from two 1-Indanone thiosemicarbazones were synthesized and tested for antiproliferative activity against the human leukemia U937 cell line. The crystal structure of [Pt(HL1)(L1)]Cl.2MeOH, where L1=1-Indanone thiosemicarbazone, was solved by X-ray diffraction. Free thiosemicarbazone ligands showed no antiproliferative effect, but the corresponding platinum(II) and palladium(II) complexes inhibited cell proliferation and induced apoptosis. Platinum(II) complexes also displayed selective apoptotic activity in U937 cells but not in peripheral blood monocytes or the human hepatocellular carcinoma HepG2 cell line used to screen for potential hepatotoxicity. Present findings show that, in U937 cells, 1-Indanone thiosemicarbazones coordinated to palladium(II) were more cytotoxic than those complexed with platinum(II), although the latter were found to be more selective for leukemic cells suggesting that they are promising compounds with potential therapeutic application against hematological malignancies.


