Licoricesaponin E2CAS# 119417-96-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 119417-96-8 | SDF | Download SDF |
| PubChem ID | 86278258 | Appearance | Powder |
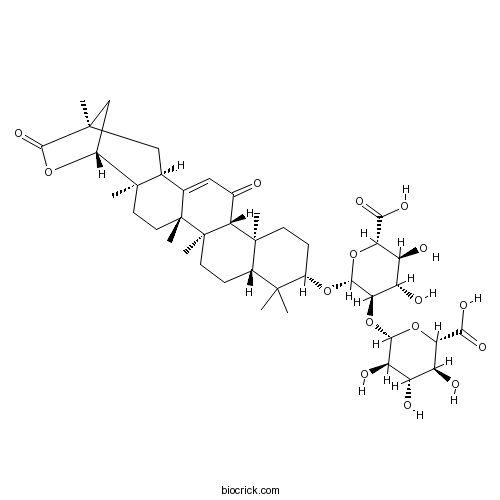
| Formula | C42H60O16 | M.Wt | 820.92 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC1(C2CCC3(C(C2(CCC1OC4C(C(C(C(O4)C(=O)O)O)O)OC5C(C(C(C(O5)C(=O)O)O)O)O)C)C(=O)C=C6C3(CCC7(C6CC8(CC7OC8=O)C)C)C)C)C | ||
| Standard InChIKey | ACCYCJOHUMRMMV-NHJGESHHSA-N | ||
| Standard InChI | InChI=1S/C42H60O16/c1-37(2)20-8-11-42(7)31(19(43)14-17-18-15-38(3)16-22(55-36(38)53)39(18,4)12-13-41(17,42)6)40(20,5)10-9-21(37)54-35-30(26(47)25(46)29(57-35)33(51)52)58-34-27(48)23(44)24(45)28(56-34)32(49)50/h14,18,20-31,34-35,44-48H,8-13,15-16H2,1-7H3,(H,49,50)(H,51,52)/t18-,20-,21-,22+,23-,24-,25-,26-,27+,28-,29-,30+,31+,34-,35+,38+,39+,40-,41+,42+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Licoricesaponin E2 displayed the inhibition on the growth of cancer cells with IC50 at 18.3-41.6 umol/L, it could significantly increase the cytotoxic activity after hydrolysis. |

Licoricesaponin E2 Dilution Calculator

Licoricesaponin E2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2181 mL | 6.0907 mL | 12.1815 mL | 24.3629 mL | 30.4536 mL |
| 5 mM | 0.2436 mL | 1.2181 mL | 2.4363 mL | 4.8726 mL | 6.0907 mL |
| 10 mM | 0.1218 mL | 0.6091 mL | 1.2181 mL | 2.4363 mL | 3.0454 mL |
| 50 mM | 0.0244 mL | 0.1218 mL | 0.2436 mL | 0.4873 mL | 0.6091 mL |
| 100 mM | 0.0122 mL | 0.0609 mL | 0.1218 mL | 0.2436 mL | 0.3045 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Topotecan hydrochloride
Catalog No.:BCN2604
CAS No.:119413-54-6
- LY2811376
Catalog No.:BCC2102
CAS No.:1194044-20-6
- Przewalskin
Catalog No.:BCN6080
CAS No.:119400-87-2
- Salviolone
Catalog No.:BCN3141
CAS No.:119400-86-1
- 2,5-Dihydroxybenzaldehyde
Catalog No.:BCN6081
CAS No.:1194-98-5
- mAChR-IN-1
Catalog No.:BCC5512
CAS No.:119391-56-9
- 2-Deacetoxytaxinine J
Catalog No.:BCN7291
CAS No.:119347-14-7
- CRANAD 2
Catalog No.:BCC6293
CAS No.:1193447-34-5
- 2-Hydroxydiplopterol
Catalog No.:BCN7290
CAS No.:1193250-54-2
- Olean-12-ene-3,24-diol
Catalog No.:BCN6079
CAS No.:119318-15-9
- 2-Benzyl-2-(dimethylamino)-4'-morpholinobutyrophenone
Catalog No.:BCC8563
CAS No.:119313-12-1
- Pinobanksin 5-methyl ether
Catalog No.:BCN7775
CAS No.:119309-36-3
- Galanin (1-30) (human)
Catalog No.:BCC6961
CAS No.:119418-04-1
- Loureirin A
Catalog No.:BCN3671
CAS No.:119425-89-7
- Loureirin B
Catalog No.:BCN5021
CAS No.:119425-90-0
- Eliprodil
Catalog No.:BCC7280
CAS No.:119431-25-3
- Fruquintinib(HMPL-013)
Catalog No.:BCC6415
CAS No.:1194506-26-7
- Meropenem trihydrate
Catalog No.:BCC4226
CAS No.:119478-56-7
- Ethyllucidone
Catalog No.:BCN6082
CAS No.:1195233-59-0
- Ceanothic acid acetate
Catalog No.:BCN6083
CAS No.:119533-63-0
- Othonnine
Catalog No.:BCN2061
CAS No.:119565-25-2
- N,N-Dimethylsphingosine
Catalog No.:BCC7959
CAS No.:119567-63-4
- 11-Hydroxygelsenicine
Catalog No.:BCN4761
CAS No.:1195760-68-9
- Dabrafenib (GSK2118436)
Catalog No.:BCC4393
CAS No.:1195765-45-7
Chemical constituents of triterpenoid saponins from Glycyrrhiza uralensis.
Chinese Traditional & Herbal Drugs, 2013, 44(12):1552-1557.
To study the chemical constituents from the roots and rhizomes of Glycyrrhiza uralensis. Methods: The compounds were separated and purified by solvent and chromatographic methods. Their structures were identified by spectroscopic techniques. Results: Fourteen triterpenoid saponins isolated from 50% ethanol extract of the roots and rhizomes of G. uralensis were identified as uralsaponin C (1), uralsaponin D (2), licorice-saponin A3 (3), uralsaponin F (4), 22β-acetoxyl-glycyrrizin (5), 24-hydroxyl-licorice-saponin E2 (6), Licoricesaponin E2 (7), licorice-saponin G2 (8), 22β-acetoxyl-glyrrhaldehyde (9), 3β-O-[β-D-glucuronopyranosyl-(1→2) - β-D-glucuronopyranosyl]-glycyrretol (10), araboglycyrrhizin (11), licorice-saponin J2 (12), glycyrrhizin (13), and glycyrrhetic acid monoglucuronide (14). Compounds 1-14 showed the cytotoxic activity against the human cancer cell lines MGC-803, SW620, and SMMC-7721 with IC50 > 100 μmol/L. The aglycones of compounds 2, 6-8, and 13 displayed the inhibition on the growth of cancer cells with IC50 at 18.3-41.6 μmol/L. Conclusion: Compound 14 is a new natural product, and compound 11 is isolated from the plant for the first time; Compounds 1-14 show no cytotoxic activity against the human cancer cell lines MGC-803, SW620, and SMMC-7721, and the aglycones of compounds 2, 6-8, and 13 could significantly increase the cytotoxic activity after hydrolysis.


