PF-04929113 (SNX-5422)Hsp90 inhibitor,potent and selective CAS# 908115-27-5 |
- CEP-32496
Catalog No.:BCC1079
CAS No.:1188910-76-0
- Sorafenib
Catalog No.:BCN2174
CAS No.:284461-73-0
- Vemurafenib (PLX4032, RG7204)
Catalog No.:BCC1269
CAS No.:918504-65-1
- BRAF inhibitor
Catalog No.:BCC1436
CAS No.:918505-61-0
- PLX-4720
Catalog No.:BCC1280
CAS No.:918505-84-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 908115-27-5 | SDF | Download SDF |
| PubChem ID | 44195571 | Appearance | Powder |
| Formula | C25H30F3N5O4 | M.Wt | 521.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
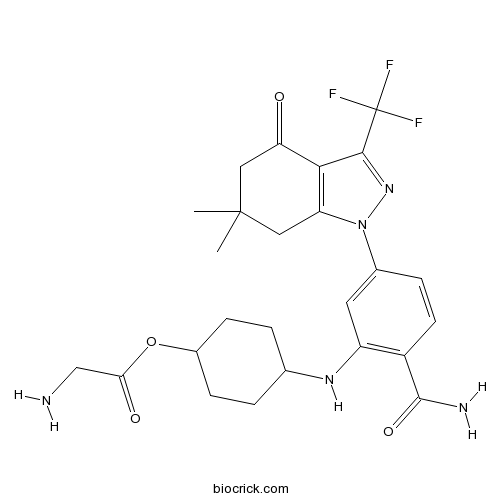
| Chemical Name | [4-[2-carbamoyl-5-[6,6-dimethyl-4-oxo-3-(trifluoromethyl)-5,7-dihydroindazol-1-yl]anilino]cyclohexyl] 2-aminoacetate | ||
| SMILES | CC1(CC2=C(C(=O)C1)C(=NN2C3=CC(=C(C=C3)C(=O)N)NC4CCC(CC4)OC(=O)CN)C(F)(F)F)C | ||
| Standard InChIKey | AVDSOVJPJZVBTC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H30F3N5O4/c1-24(2)10-18-21(19(34)11-24)22(25(26,27)28)32-33(18)14-5-8-16(23(30)36)17(9-14)31-13-3-6-15(7-4-13)37-20(35)12-29/h5,8-9,13,15,31H,3-4,6-7,10-12,29H2,1-2H3,(H2,30,36) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | PF-04929113 is a potent and selective inhibitor of Hsp90 with IC50 value of 50 nM. | |||||
| Targets | Hsp90 | |||||
| IC50 | 50 nM | |||||

PF-04929113 (SNX-5422) Dilution Calculator

PF-04929113 (SNX-5422) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9175 mL | 9.5877 mL | 19.1755 mL | 38.3509 mL | 47.9386 mL |
| 5 mM | 0.3835 mL | 1.9175 mL | 3.8351 mL | 7.6702 mL | 9.5877 mL |
| 10 mM | 0.1918 mL | 0.9588 mL | 1.9175 mL | 3.8351 mL | 4.7939 mL |
| 50 mM | 0.0384 mL | 0.1918 mL | 0.3835 mL | 0.767 mL | 0.9588 mL |
| 100 mM | 0.0192 mL | 0.0959 mL | 0.1918 mL | 0.3835 mL | 0.4794 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PF-04929113 is an inhibitor of Hsp90 [1].
PF-04929113 is a water soluble and orally bioavailable prodrug of PF-04928473. It is rapidly absorbed and converted into PF-04928473 after oral administration. In mice bearing BT-474 tumor xenografts, treatment of PF-04928473 causes the degradation of the HER2 client protein. And no obvious toxicity is observed when the dose of PF-04928473 is up to 150mg/kg. Treatment of 100mg/kg PF-04928473 results in complete tumor growth inhibition and in some mice partial tumor regressions. PF-04928473 also shows significant antitumor activity in mice with H1650 xenografts. In addition, as an inhibitor of Hsp90, PF-04928473 is reported to inhibit p-ERK and p-Akt, decrease CD31+ cells and MVD as well as a have an effect on angiogenesis in vivo [1, 2].
References:
[1] Chandarlapaty S, Sawai A, Ye Q, Scott A, Silinski M, Huang K, Fadden P, Partdrige J, Hall S, Steed P, Norton L, Rosen N, Solit DB. SNX2112, a synthetic heat shock protein 90 inhibitor, has potent antitumor activity against HER kinase-dependent cancers. Clin Cancer Res. 2008 Jan 1;14(1):240-8.
[2] Okawa Y, Hideshima T, Steed P, Vallet S, Hall S, Huang K, Rice J, Barabasz A, Foley B, Ikeda H, Raje N, Kiziltepe T, Yasui H, Enatsu S, Anderson KC. SNX-2112, a selective Hsp90 inhibitor, potently inhibits tumor cell growth, angiogenesis, and osteoclastogenesis in multiple myeloma and other hematologic tumors by abrogating signaling via Akt and ERK. Blood. 2009 Jan 22;113(4):846-55.
- SNX-2112
Catalog No.:BCC2132
CAS No.:908112-43-6
- 6,8-Di-O-methylcitreoisocoumarin
Catalog No.:BCN7380
CAS No.:908098-80-6
- Atosiban
Catalog No.:BCC5314
CAS No.:90779-69-4
- Musellactone
Catalog No.:BCN7183
CAS No.:907583-51-1
- (S)-4-Benzyl-2-oxazolidinone
Catalog No.:BCC8401
CAS No.:90719-32-7
- Ethyl beta-carboline-1-propionate
Catalog No.:BCN1311
CAS No.:90686-24-1
- 5-Hydroxysophoranone
Catalog No.:BCN6842
CAS No.:90686-12-7
- Methyl 1,4-bisglucosyloxy-3-prenyl-2-naphthoate
Catalog No.:BCN7597
CAS No.:90685-26-0
- SCH 546738
Catalog No.:BCC4110
CAS No.:906805-42-3
- Parvifolixanthone B
Catalog No.:BCN7421
CAS No.:906794-57-8
- Parvifolixanthone A
Catalog No.:BCN7354
CAS No.:906794-56-7
- AN-2728
Catalog No.:BCC1361
CAS No.:906673-24-3
- Cyclo(Ile-Ala)
Catalog No.:BCN2429
CAS No.:90821-99-1
- Goshonoside F1
Catalog No.:BCN6444
CAS No.:90851-24-4
- Goshonoside F5
Catalog No.:BCN6442
CAS No.:90851-28-8
- ent-Labda-8(17),13E-diene-3beta,15,18-triol
Catalog No.:BCN7662
CAS No.:90851-50-6
- Ptelatoside B
Catalog No.:BCN4451
CAS No.:90852-99-6
- Aprotinin
Catalog No.:BCC1220
CAS No.:9087-70-1
- α-helical CRF 9-41
Catalog No.:BCC5727
CAS No.:90880-23-2
- M871
Catalog No.:BCC5930
CAS No.:908844-75-7
- Broussonin E
Catalog No.:BCN4452
CAS No.:90902-21-9
- Erythrocentauric acid
Catalog No.:BCN7683
CAS No.:90921-13-4
- Cl-HIBO
Catalog No.:BCC7147
CAS No.:909400-43-7
- α-CGRP (human)
Catalog No.:BCC5962
CAS No.:90954-53-3
A phase I study of PF-04929113 (SNX-5422), an orally bioavailable heat shock protein 90 inhibitor, in patients with refractory solid tumor malignancies and lymphomas.[Pubmed:21908572]
Clin Cancer Res. 2011 Nov 1;17(21):6831-9.
PURPOSE: To determine the maximum tolerated dose (MTD), toxicities, and pharmacokinetic/pharmacodynamic profile of the Hsp90 inhibitor PF-04929113 (SNX-5422) in patients with advanced solid tumors and lymphomas. METHODS: This was a single-institution, phase I, dose-escalation study of PF-04929113 administered twice weekly. Endpoints included determination of dose-limiting toxicities (DLT), MTD, the safety profile of PF-04929113, pharmacodynamic assessment of PF-04929113 on Hsp70 induction, pharmacokinetic analysis of PF-04928473 (SNX-2112) and its prodrug PF-04929113, and assessment of response. RESULTS: Thirty-three patients with advanced malignancies were treated. Dose escalation was continued up to 177 mg/m(2) administered orally twice a week. One DLT (nonseptic arthritis) was noted. No grade 4 drug-related adverse events were seen; grade 3 adverse events included diarrhea (9%), nonseptic arthritis (3%), aspartate aminotransferase elevation (3%), and thrombocytopenia (3%). No objective responses were seen in 32 evaluable patients. Fifteen patients (47%) had stable disease; 17 patients (53%) had progressive disease. Pharmacokinetic data revealed rapid absorption, hepatic, and extrahepatic clearance, extensive tissue binding, and almost linear pharmacokinetics of the active drug PF-04928473. Pharmacodynamic studies confirmed inhibition of Hsp90 and a linear correlation between pharmacokinetic parameters and Hsp70 induction. CONCLUSIONS: PF-04929113 administered orally twice a week is well tolerated and inhibits its intended target Hsp90. No objective responses were seen, but long-lasting stabilizations were obtained. Although no clinically significant drug-related ocular toxicity was seen in this study, the development of PF-04929113 has been discontinued because of ocular toxicity seen in animal models and in a separate phase I study.


