2-HydroxyquinoxalineCAS# 1196-57-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1196-57-2 | SDF | Download SDF |
| PubChem ID | 14526 | Appearance | Powder |
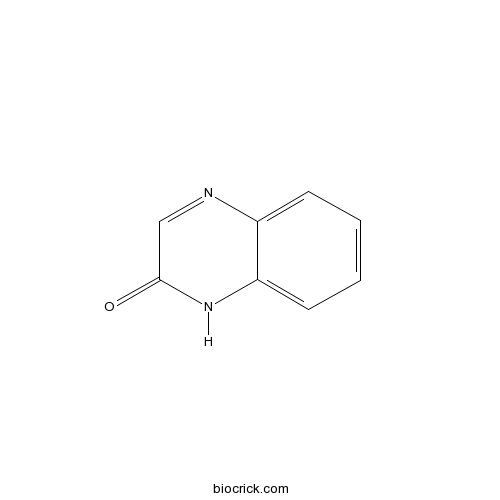
| Formula | C8H6N2O | M.Wt | 146 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1H-quinoxalin-2-one | ||
| SMILES | C1=CC=C2C(=C1)NC(=O)C=N2 | ||
| Standard InChIKey | FFRYUAVNPBUEIC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H6N2O/c11-8-5-9-6-3-1-2-4-7(6)10-8/h1-5H,(H,10,11) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2-Hydroxyquinoxaline Dilution Calculator

2-Hydroxyquinoxaline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.8493 mL | 34.2466 mL | 68.4932 mL | 136.9863 mL | 171.2329 mL |
| 5 mM | 1.3699 mL | 6.8493 mL | 13.6986 mL | 27.3973 mL | 34.2466 mL |
| 10 mM | 0.6849 mL | 3.4247 mL | 6.8493 mL | 13.6986 mL | 17.1233 mL |
| 50 mM | 0.137 mL | 0.6849 mL | 1.3699 mL | 2.7397 mL | 3.4247 mL |
| 100 mM | 0.0685 mL | 0.3425 mL | 0.6849 mL | 1.3699 mL | 1.7123 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 7-Ethyl-10-Hydroxy-Camptothecin
Catalog No.:BCN8386
CAS No.:119577-28-5
- Dabrafenib Mesylate (GSK-2118436)
Catalog No.:BCC1513
CAS No.:1195768-06-9
- Dabrafenib (GSK2118436)
Catalog No.:BCC4393
CAS No.:1195765-45-7
- 11-Hydroxygelsenicine
Catalog No.:BCN4761
CAS No.:1195760-68-9
- N,N-Dimethylsphingosine
Catalog No.:BCC7959
CAS No.:119567-63-4
- Othonnine
Catalog No.:BCN2061
CAS No.:119565-25-2
- Ceanothic acid acetate
Catalog No.:BCN6083
CAS No.:119533-63-0
- Ethyllucidone
Catalog No.:BCN6082
CAS No.:1195233-59-0
- Meropenem trihydrate
Catalog No.:BCC4226
CAS No.:119478-56-7
- Fruquintinib(HMPL-013)
Catalog No.:BCC6415
CAS No.:1194506-26-7
- Eliprodil
Catalog No.:BCC7280
CAS No.:119431-25-3
- Loureirin B
Catalog No.:BCN5021
CAS No.:119425-90-0
- GSK2190915 sodium salt
Catalog No.:BCC5588
CAS No.:1196070-26-4
- PF-3845
Catalog No.:BCC2326
CAS No.:1196109-52-0
- Olprinone Hydrochloride
Catalog No.:BCC1821
CAS No.:119615-63-3
- Sulfo-NHS-Biotin
Catalog No.:BCC3576
CAS No.:119616-38-5
- Arecaidine but-2-ynyl ester tosylate
Catalog No.:BCC6627
CAS No.:119630-77-2
- Naloxone benzoylhydrazone
Catalog No.:BCC5757
CAS No.:119630-94-3
- Moguisteine
Catalog No.:BCC4925
CAS No.:119637-67-1
- Yucalexin P-17
Catalog No.:BCN6595
CAS No.:119642-82-9
- Amadacycline methanesulfonate
Catalog No.:BCC1356
CAS No.:1196800-40-4
- 3',4'-Dihydroxyacetophenone
Catalog No.:BCN4775
CAS No.:1197-09-7
- Tranexamic acid
Catalog No.:BCN2710
CAS No.:1197-18-8
- 4-Aminophenylacetic acid
Catalog No.:BCC8687
CAS No.:1197-55-3
Biodegradation of organophosphate pesticide quinalphos by Ochrobactrum sp. strain HZM.[Pubmed:25155583]
J Appl Microbiol. 2014 Nov;117(5):1283-92.
AIMS: Isolation and identification of bacteria capable of degrading organophosphate pesticide quinalphos and elucidation of its biodegradative pathway. METHODS AND RESULTS: A bacterium capable of degrading organophosphate pesticides was isolated from the pesticide-contaminated soil samples by selective enrichment on quinalphos (QP) as a sole source of carbon and energy. The bacterial strain was identified as Ochrobactrum sp. strain HZM on the basis of its morphological and biochemical characteristics and by phylogenetic analysis based on 16S rRNA gene sequences. The organism utilized various organophosphate pesticides such as quinalphos, profenofos, parathion-methyl and chlorpyrifos as growth substrates. Response surface methodology (RSM) showed optimum conditions for quinalphos degradation at pH 7 and 27 degrees C. 2-Hydroxyquinoxaline and diethyl phosphate were identified as metabolites of quinalphos degradation by HPLC and GC-MS analysis. Cell-free extract of Ochrobactrum sp. strain HZM grown on quinalphos contained the quinalphos hydrolase activity. CONCLUSIONS: A bacterial strain capable of degrading quinalphos was isolated and identified as Ochrobactrum sp. strain HZM. The organism utilized organophosphate pesticides quinalphos, profenofos, parathion-methyl and chlorpyrifos as carbon sources. The organism degraded quinalphos by hydrolysis to yield 2-Hydroxyquinoxaline and diethyl phosphate which were further utilized as carbon sources. SIGNIFICANCE AND IMPACT OF THE STUDY: The isolated bacterium Ochrobactrum sp. strain HZM was versatile in degrading various organophosphate pesticides. There was complete mineralization of quinalphos by Ochrobactrum sp. This strain could potentially be useful in the bioremediation of soil and water contaminated with toxic organophosphate pesticides.
Biodegradation of 2-hydroxyquinoxaline (2-HQ) by Bacillus sp.[Pubmed:24953941]
J Hazard Mater. 2014 Aug 15;278:100-7.
An aerobic Gram +ve bacterial strain capable of utilizing 2-Hydroxyquinoxaline (2-HQ) as sole source of carbon and energy was isolated from Chrysanthemum indicum Indian agricultural soil and named as HQ2. On the basis of morphology, physico-biochemical characteristics and 16S rRNA sequence analysis, strain HQ2 was identified as Bacillus sp. The generation time of Bacillus sp. in log phase during growth on 2-HQ is 0.79 h or 47.4 min. The optimal conditions for 2-HQ degradation by Bacillus sp. were inoculum density of 1.0 OD, pH of 6-8, temperature of 37-45 degrees C and 2-HQ concentration of 500 ppm. Among the additional carbon and nitrogen sources, carbon sources did not influence the degradation rate of 2-HQ, but nitrogen sources-yeast extract marginally enhanced the rate of degradation of 2-HQ. GC-MS analysis of the culture Bacillus sp. grown on 2-HQ indicated the formation of dimers from 2 molecules of 2-Hydroxyquinoxaline. The formation of dimer for degradation of 2-HQ by the culture appears to be the first report to our scientific knowledge.


