Olprinone HydrochloridePhosphodiesterase 3 (PDE3) inhibitor, selective CAS# 119615-63-3 |
- PD 123319 ditrifluoroacetate
Catalog No.:BCC1841
CAS No.:136676-91-0
- Irbesartan
Catalog No.:BCC2560
CAS No.:138402-11-6
- AVE 0991
Catalog No.:BCC4032
CAS No.:304462-19-9
- Tranilast
Catalog No.:BCC2514
CAS No.:53902-12-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 119615-63-3 | SDF | Download SDF |
| PubChem ID | 115227 | Appearance | Powder |
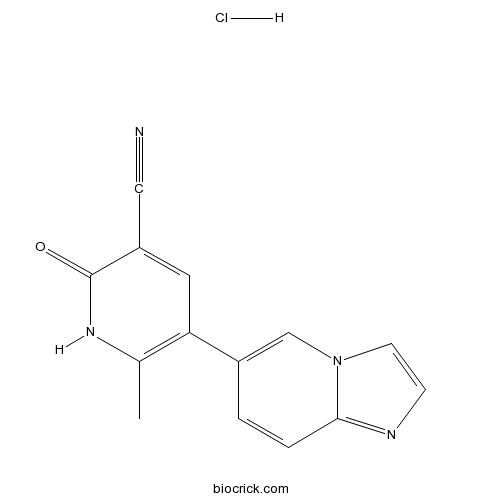
| Formula | C14H11ClN4O | M.Wt | 286.72 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 7.69 mg/mL (26.82 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 5-imidazo[1,2-a]pyridin-6-yl-6-methyl-2-oxo-1H-pyridine-3-carbonitrile;hydrochloride | ||
| SMILES | CC1=C(C=C(C(=O)N1)C#N)C2=CN3C=CN=C3C=C2.Cl | ||
| Standard InChIKey | PWTBMBAQRAOAFF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H10N4O.ClH/c1-9-12(6-11(7-15)14(19)17-9)10-2-3-13-16-4-5-18(13)8-10;/h2-6,8H,1H3,(H,17,19);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Olprinone Hydrochloride Dilution Calculator

Olprinone Hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4877 mL | 17.4386 mL | 34.8772 mL | 69.7545 mL | 87.1931 mL |
| 5 mM | 0.6975 mL | 3.4877 mL | 6.9754 mL | 13.9509 mL | 17.4386 mL |
| 10 mM | 0.3488 mL | 1.7439 mL | 3.4877 mL | 6.9754 mL | 8.7193 mL |
| 50 mM | 0.0698 mL | 0.3488 mL | 0.6975 mL | 1.3951 mL | 1.7439 mL |
| 100 mM | 0.0349 mL | 0.1744 mL | 0.3488 mL | 0.6975 mL | 0.8719 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Olprinone is a selective phosphodiesterase 3 (PDE3) inhibitor. Olprinone is used as cardiotonic agent with positive inotropic and vasodilating effects. Olprinone has been reported to improve microcirculation and attenuate inflammation. Olprinone is often used to increase cardiac output after cardiopulmonary bypass (CPB). Olprinone was infused at a rate of 0.2 μg/kg/min when weaning from CPB was started. Olprinone has also shown potent antioxidative and anti-inflammatory effects in the meconium-induced oxidative lung injury.
- PF-3845
Catalog No.:BCC2326
CAS No.:1196109-52-0
- GSK2190915 sodium salt
Catalog No.:BCC5588
CAS No.:1196070-26-4
- 2-Hydroxyquinoxaline
Catalog No.:BCC8577
CAS No.:1196-57-2
- 7-Ethyl-10-Hydroxy-Camptothecin
Catalog No.:BCN8386
CAS No.:119577-28-5
- Dabrafenib Mesylate (GSK-2118436)
Catalog No.:BCC1513
CAS No.:1195768-06-9
- Dabrafenib (GSK2118436)
Catalog No.:BCC4393
CAS No.:1195765-45-7
- 11-Hydroxygelsenicine
Catalog No.:BCN4761
CAS No.:1195760-68-9
- N,N-Dimethylsphingosine
Catalog No.:BCC7959
CAS No.:119567-63-4
- Othonnine
Catalog No.:BCN2061
CAS No.:119565-25-2
- Ceanothic acid acetate
Catalog No.:BCN6083
CAS No.:119533-63-0
- Ethyllucidone
Catalog No.:BCN6082
CAS No.:1195233-59-0
- Meropenem trihydrate
Catalog No.:BCC4226
CAS No.:119478-56-7
- Sulfo-NHS-Biotin
Catalog No.:BCC3576
CAS No.:119616-38-5
- Arecaidine but-2-ynyl ester tosylate
Catalog No.:BCC6627
CAS No.:119630-77-2
- Naloxone benzoylhydrazone
Catalog No.:BCC5757
CAS No.:119630-94-3
- Moguisteine
Catalog No.:BCC4925
CAS No.:119637-67-1
- Yucalexin P-17
Catalog No.:BCN6595
CAS No.:119642-82-9
- Amadacycline methanesulfonate
Catalog No.:BCC1356
CAS No.:1196800-40-4
- 3',4'-Dihydroxyacetophenone
Catalog No.:BCN4775
CAS No.:1197-09-7
- Tranexamic acid
Catalog No.:BCN2710
CAS No.:1197-18-8
- 4-Aminophenylacetic acid
Catalog No.:BCC8687
CAS No.:1197-55-3
- 5-Methylfurmethiodide
Catalog No.:BCC6707
CAS No.:1197-60-0
- PF-05212384 (PKI-587)
Catalog No.:BCC4987
CAS No.:1197160-78-3
- Sutchuenmedin A
Catalog No.:BCN6854
CAS No.:1197194-31-2
[Successful management of a patient for cardiac surgery with difficulty in weaning from cardiopulmonary bypass by using both isosorbide dinitrate and olprinone hydrochloride].[Pubmed:15298245]
Masui. 2004 Jul;53(7):777-81.
A 57-year-old man with mitral stenosis underwent mitral valve plasty under general anesthesia. He had a history of cerebral infarction. Although he was with atrial fibrillation, his left ventricular function was good. Preoperative coronary angiography revealed no significant coronary stenosis. Induction of anesthesia and the surgical procedure had been uneventful, but the patient had difficulty to wean the patient from cardiopulmonary bypass because of unexpected low cardiac output syndrome. O1-prinone hydrochloride, a newly developed phosphodiesterase III inhibitor, was initiated in addition to high doses of dopamine and dobutamine. This increased the amplitude of the electrocardiogram and caused ST elevation of the lead II. A full dose of isosorbide dinitrate was administered intravenously to differentiate coronary artery spasm from coronary air embolism. This drastically improved the ventricular function and mixed venous oxygen saturation, and weaning from CPB was finally accomplished. The heart showed hypercontraction and inotropes were tapered gradually without further cardiac events. Although there are various etiologies for low cardiac output syndrome after CPB, the possibility of myocardial ischemia must be the first consideration. Full pharmacological support must be tried before initiating a mechanical assist modality. Coronary dilators, nitrates in particular, and phosphodiesterase III inhibitors are promising agents in such cases.
Pretreatment with olprinone hydrochloride, a phosphodiesterase III inhibitor, attenuates lipopolysaccharide-induced lung injury via an anti-inflammatory effect.[Pubmed:17434327]
Pulm Pharmacol Ther. 2008;21(1):166-71.
PURPOSE: Acute respiratory distress syndrome is characterized by neutrophil accumulation in the lungs and the activation of several cytokines produced by macrophages. Olprinone Hydrochloride, a specific phosphodiesterase III inhibitor, has anti-inflammatory effects and inhibits the activation of macrophages, in addition to its inotropic and vasodilatory effects. The purpose of this study was to examine the beneficial effects of olprinone on lipopolysaccharide (LPS)-induced pulmonary inflammation. MATERIALS AND METHODS: Lung inflammation was produced by intravenous LPS injection into rats. The rats were divided into four groups: a vehicle group in which normal saline was injected, an olprinone group in which olprinone was injected at a dose of 0.2mg/kg, a dexamethasone group in which dexamethasone was injected at a dose of 5mg/kg, and a control group. In each group, drug was injected intraperitoneally 30 min before the intravenous administration of LPS. The blood was obtained at 1h and then animals were sacrificed at 6h and blood and lung specimen were obtained for cytokine analysis and pathological examination. On another set of experiment, bronchioloalveolar lavage (BAL) was performed for cytokine analysis of BAL fluid. The macrophages isolated from normal rat by BAL were cultured in vitro with the presence of LPS and olprinone or dexamethasone, and supernatant was collected. The levels of several cytokines in the serum, in the BAL fluid, and in the culture supernatant were determined. RESULTS: The animals injected with LPS were found to have an influx of neutrophils in the lungs, and inflammatory cytokines, such as TNF-alpha and IL-6, and anti-inflammatory cytokine IL-10 were produced. Pretreatment with olprinone or dexamethasone significantly inhibited the LPS-induced neutrophil influx into the lungs, suppressed inflammatory cytokines TNF-alpha and IL-6. The level of anti-inflammatory cytokine IL-10 increased in an olprinone group. The inhibition of TNF-alpha and IL-6, and the augmentation of IL-10 release were also observed in in vitro culture of isolated rat alveolar macrophages when olprinone (10(-5)mol/ml) and LPS (10 microg/ml) were cultured together. However, the level of IL-10 in serum and culture supernatant was suppressed in a dexamesathone group. CONCLUSION: LPS-induced lung inflammation is strongly inhibited by olprinone accompanying the enhancement of IL-10 and the inhibition of inflammatory cytokines. Results of the in vitro experiment suggest that alveolar macrophages may play an important role in ameliorating LPS-induced lung inflammation and the mechanism of its effect is different from that of steroid.
[Effects of olprinone hydrochloride in patients undergoing off-pump coronary artery bypass grafting].[Pubmed:16491891]
Masui. 2006 Feb;55(2):158-63.
BACKGROUND: Olprinone Hydrochloride (OLP) is a new phosphodiesterase III inhibitor with positive inotropic and vasodilator properties. It is characterized by strong inotropic effect and relatively weak vasodilating effect. Although the method for administration of OLP has been reasonably optimized, no studies have examined the administration mode for patients with off-pump coronary artery bypass grafting (OPCAB). METHODS: After the start of the operation, 12 patients received OLP 0.2 microg x kg(-1) x min(-1) (OLP group) and 14 patients received normal saline (control group). Catecholamines were concomitantly administered to maintain systolic pressure > or = 100 mmHg and cardiac index (CI) > or = 2.5 l x min(-1) x m(-2). Hemodymanic parameters were measured after induction of anesthesia, at the administration of heparin, immediately after coronary artery anastomosis, at the end of the operation, and 3 h and 6 h after the operation. RESULTS: Systolic and mean arterial pressures did not differ between the two groups. CI was significantly increased, and SVRI was significantly decreased after administration of heparin, immediately after coronary artery anastomosis, at the end of the operation and 3 h after the operation in the OLP group. OLP reduced the number of patients requiring dopamine and dobutamine compared with the control group. CONCLUSIONS: The study showed that OLP administrated during OPCAB may have improved hemodynamics in patients for OPCAB.
[Successful anesthetic management of three patients with cardiac dysfunction for non-cardiac surgery using olprinone hydrochloride].[Pubmed:16026056]
Masui. 2005 Jul;54(7):757-61.
Non-cardiac surgery presents significant risks to patients with cardiac dysfunction. The relative safety of different anesthetic techniques has been studied without mentioning any clear indication. The depression of myocardial contractility by anesthetic agents limits their use in patients with cardiac dysfunction, especially for induction of anesthesia. We used Olprinone Hydrochloride perioperatively in the anesthetic management of three patients. In all cases, anesthetic induction, intraoperative course and the postoperative period proceeded uneventfully. We consider that perioperative use of continuous Olprinone Hydrochloride infusion may be suitable for patients with cardiac dysfunction for non-cardiac surgery.


