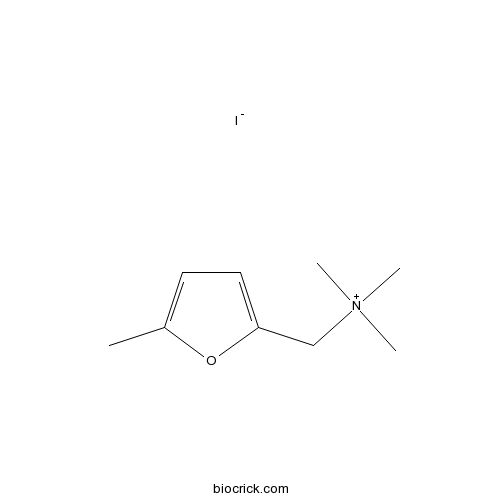
5-MethylfurmethiodidePotent muscarinic agonist CAS# 1197-60-0 |
- Koumine
Catalog No.:BCN6190
CAS No.:1358-76-5
- Byakangelicol
Catalog No.:BCN5015
CAS No.:26091-79-2
- Obtusifolin
Catalog No.:BCN2537
CAS No.:477-85-0
- Gelsemine
Catalog No.:BCN5804
CAS No.:509-15-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1197-60-0 | SDF | Download SDF |
| PubChem ID | 70973 | Appearance | Powder |
| Formula | C9H16INO | M.Wt | 281.14 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Methylfutrethonium iodide | ||
| Solubility | Soluble to 100 mM in DMSO | ||
| Chemical Name | trimethyl-[(5-methylfuran-2-yl)methyl]azanium;iodide | ||
| SMILES | CC1=CC=C(O1)C[N+](C)(C)C.[I-] | ||
| Standard InChIKey | IHTCZSNKQINGDD-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C9H16NO.HI/c1-8-5-6-9(11-8)7-10(2,3)4;/h5-6H,7H2,1-4H3;1H/q+1;/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A potent muscarinic agonist. |

5-Methylfurmethiodide Dilution Calculator

5-Methylfurmethiodide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5569 mL | 17.7847 mL | 35.5695 mL | 71.1389 mL | 88.9237 mL |
| 5 mM | 0.7114 mL | 3.5569 mL | 7.1139 mL | 14.2278 mL | 17.7847 mL |
| 10 mM | 0.3557 mL | 1.7785 mL | 3.5569 mL | 7.1139 mL | 8.8924 mL |
| 50 mM | 0.0711 mL | 0.3557 mL | 0.7114 mL | 1.4228 mL | 1.7785 mL |
| 100 mM | 0.0356 mL | 0.1778 mL | 0.3557 mL | 0.7114 mL | 0.8892 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-Aminophenylacetic acid
Catalog No.:BCC8687
CAS No.:1197-55-3
- Tranexamic acid
Catalog No.:BCN2710
CAS No.:1197-18-8
- 3',4'-Dihydroxyacetophenone
Catalog No.:BCN4775
CAS No.:1197-09-7
- Amadacycline methanesulfonate
Catalog No.:BCC1356
CAS No.:1196800-40-4
- Yucalexin P-17
Catalog No.:BCN6595
CAS No.:119642-82-9
- Moguisteine
Catalog No.:BCC4925
CAS No.:119637-67-1
- Naloxone benzoylhydrazone
Catalog No.:BCC5757
CAS No.:119630-94-3
- Arecaidine but-2-ynyl ester tosylate
Catalog No.:BCC6627
CAS No.:119630-77-2
- Sulfo-NHS-Biotin
Catalog No.:BCC3576
CAS No.:119616-38-5
- Olprinone Hydrochloride
Catalog No.:BCC1821
CAS No.:119615-63-3
- PF-3845
Catalog No.:BCC2326
CAS No.:1196109-52-0
- GSK2190915 sodium salt
Catalog No.:BCC5588
CAS No.:1196070-26-4
- PF-05212384 (PKI-587)
Catalog No.:BCC4987
CAS No.:1197160-78-3
- Sutchuenmedin A
Catalog No.:BCN6854
CAS No.:1197194-31-2
- UNC 0224
Catalog No.:BCC2430
CAS No.:1197196-48-7
- 2-Epitormentic acid
Catalog No.:BCN6084
CAS No.:119725-19-8
- Fupenzic acid
Catalog No.:BCN6085
CAS No.:119725-20-1
- Baohuoside VII
Catalog No.:BCN2889
CAS No.:119730-89-1
- TGR5 Receptor Agonist
Catalog No.:BCC4195
CAS No.:1197300-24-5
- Sazetidine A dihydrochloride
Catalog No.:BCC7468
CAS No.:1197329-42-2
- SDZ 205-557 hydrochloride
Catalog No.:BCC7246
CAS No.:1197334-02-3
- SB 206553 hydrochloride
Catalog No.:BCC7143
CAS No.:1197334-04-5
- Schizanthine G
Catalog No.:BCN1938
CAS No.:119736-74-2
- Schizanthine M
Catalog No.:BCN1939
CAS No.:119736-78-6
Effects of the muscarinic agonist, 5-methylfurmethiodide, on contraction and electrophysiology of Ascaris suum muscle.[Pubmed:18206155]
Int J Parasitol. 2008 Jul;38(8-9):945-57.
Contraction and electrophysiological effects of 5-Methylfurmethiodide (MFI), a selective muscarinic agonist in mammals, were tested on Ascaris suum muscle strips. In a contraction assay, MFI produced weak contraction and was less potent than levamisole and acetylcholine. Atropine (3microM) a non-selective muscarinic antagonist in mammalian preparations, did not affect contractions produced by MFI. Mecamylamine (3microM) a nicotinic antagonist in A. suum preparations, blocked the MFI contractions indicating that MFI had weak nicotinic agonist actions. In two-micropipette current-clamp experiments MFI, at concentrations greater than 10microM, produced concentration-dependent depolarizations and small increases in membrane conductance. The depolarizing effects were not abolished by perfusing the preparation in a calcium-free Ascaris Ringer solution to block synaptic transmission, suggesting that MFI effects were mediated by receptors on the muscle and were calcium-independent. A high concentration of mecamylamine, 30microM, only reduced the depolarizing responses by 42%, indicating that MFI also had effects on non-nicotinic receptors. Three non-nicotinic effects in the presence of 30microM mecamylamine were identified using voltage-clamp techniques: (i) MFI produced opening of mecamylamine-resistant non-selective-cation channel currents; (ii) MFI inhibited opening of voltage-activated potassium currents; and (iii) MFI increased the threshold of voltage-activated calcium currents. We suggest that a drug that is more selective for voltage-activated potassium currents, without effects on other channels like MFI, may be exploited pharmacologically as a novel anthelmintic or as an agent to potentiate the action of levamisole. In a larval migration assay we demonstrated that 4-aminopyridine (4-AP: a potassium channel blocker) potentiated the effects of levamisole but MFI did not.
A pharmacological study of the responses induced by muscarinic agonists on the isolated superior cervical ganglion of the guinea-pig.[Pubmed:2289527]
Eur J Pharmacol. 1990 Sep 21;186(2-3):257-65.
We have studied the muscarinic agonist induced responses on the guinea-pig superior cervical ganglion in vitro, as recorded from the internal carotid nerve using a grease-gap. The principal response was a depolarization, but a small hyperpolarizing response could be revealed under certain conditions. We determined the pA2 of a number of muscarinic antagonists against the muscarine induced depolarization. Four selective antagonists and atropine appeared to act competitively. The rank order of their pA2s was 4-DAMP (8.5), atropine (8.4), pirenzepine (8.0), methoctramine (7.2) and AF-DX 116 (6.3). In addition to muscarine, we assessed the potency and relative maximum response of nine other muscarinic compounds to depolarize this preparation: carbachol, 5-methylfurmethide, oxotremorine, oxotremorine-M, pilocarpine, RS 86, AF102B and two novel compounds L-670548 and L-679512. L-670548 was the most potent and AF102B was the least potent agonist tested. Only AF102B evoked a maximum depolarization that was significantly smaller than muscarine. A hyperpolarizing response to carbachol (1 microM) could be recorded when the superfusing medium contained 0.3 microM pirenzepine and only 0.1 mM CaCl2 (cf. usual 2.5 mM). This response was relatively small compared to that evoked on the superior cervical ganglion of the rat. It was blocked by the cardioselective antagonists methoctramine (0.1-0.3 microM) and AF-DX 116 (0.3-1.0 microM). Of the 10 agonists tested, only carbachol, oxotremorine and oxotremorine-M reproducibly evoked a hyperpolarizing response. It was concluded that muscarinic agonists can induce a depolarization of the guinea-pig superior cervical ganglion mediated by M1 receptors. The activation of cardiac-like M2 receptors resulted in a hyperpolarizing response that was relatively small.
Pharmacological differences between two muscarinic responses of the rat superior cervical ganglion in vitro.[Pubmed:3427281]
Br J Pharmacol. 1987 Dec;92(4):817-26.
1 Pharmacological differences have been observed between the muscarinic agonist-induced depolarizing and hyperpolarizing responses of the rat isolated superior cervical ganglion. 2 Pirenzepine (0.3 microM) selectively reduced the depolarizing response and unmasked the hyperpolarizing response. No such selectivity was observed with a concentration of N-methylatropine which was equipotent with pirenzepine in antagonizing the depolarizing response. 3 The neuromuscular blocking agents gallamine (10 microM) and pancuronium (3 microM) exhibited the oppositive selectivity to pirenzepine, both dramatically reduced the hyperpolarization but only slightly antagonized the depolarization. 4 The potencies of a range of agonists in evoking the depolarizing and hyperpolarizing responses, the latter in the presence of 0.3 microM pirenzepine, have been determined. Methylfurmethide failed to hyperpolarize the ganglion at concentrations which evoked maximal depolarizations. 5 The muscarinic hyperpolarization did not appear to be mediated by the secondary release of catecholamines. 6 It was concluded that the two muscarinic responses on the rat superior cervical ganglion, the slow depolarization and faster hyperpolarization, are mediated by different muscarinic receptor subtypes.


