ByakangelicolCAS# 26091-79-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 26091-79-2 | SDF | Download SDF |
| PubChem ID | 3055167 | Appearance | White powder |
| Formula | C17H16O6 | M.Wt | 316.31 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Synonyms | Biacangelicol | ||
| Solubility | Soluble in chloroform, methanol and pyridine | ||
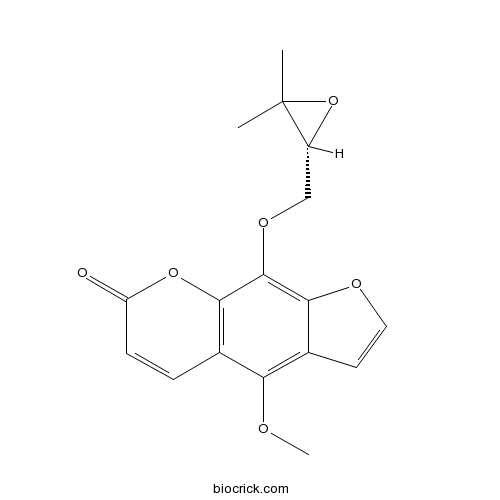
| Chemical Name | 9-[[(2R)-3,3-dimethyloxiran-2-yl]methoxy]-4-methoxyfuro[3,2-g]chromen-7-one | ||
| SMILES | CC1(C(O1)COC2=C3C(=C(C4=C2OC(=O)C=C4)OC)C=CO3)C | ||
| Standard InChIKey | ORBITTMJKIGFNH-LLVKDONJSA-N | ||
| Standard InChI | InChI=1S/C17H16O6/c1-17(2)11(23-17)8-21-16-14-10(6-7-20-14)13(19-3)9-4-5-12(18)22-15(9)16/h4-7,11H,8H2,1-3H3/t11-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Byakangelicol exhibits hepatoprotective activities on tacrine-induced cytotoxicity in Hep G2 cells, with EC(50) values of 112.7 +/- 5.35 microM. Byakangelicol may have therapeutic potential as an anti-inflammatory drug on airway inflammation, it can inhibit IL-1beta-induced PGE2 release in A549 cells; this inhibition may be mediated by suppression of COX-2 expression and the activity of COX-2 enzyme, it also can inhibit P-gp expressed. Byakangelicol shows a significant inhibition on the proliferation of cultured human tumor cells. |
| Targets | COX | PGE | IL Receptor | MAPK | p65 | P-gp | NF-kB |
| In vitro | Byakangelicol, isolated from Angelica dahurica, inhibits both the activity and induction of cyclooxygenase-2 in human pulmonary epithelial cells.[Pubmed: 12356282]J Pharm Pharmacol. 2002 Sep;54(9):1271-8.We examined the inhibitory mechanism of Byakangelicol, isolated from Angelica dahurica, on interleukin-1beta (IL-1beta)-induced cyclooxygenase-2 (COX-2) expression and prostaglandin E2 (PGE2) release in human pulmonary epithelial cell line (A549).
Antiproliferative effect of furanocoumarins from the root of Angelica dahurica on cultured human tumor cell lines.[Pubmed: 17143927 ]Phytother Res. 2007 Mar;21(3):288-90.A bioassay-guided fractionation of the root extract of Angelica dahurica (Umbelliferae) led to the isolation of six furanocoumarins as active ingredients responsible for the antitumoral property.
|
| Kinase Assay | Inhibitory effects of furanocoumarin derivatives in Kampo extract medicines on P-glycoprotein at the blood-brain barrier.[Pubmed: 21804213]Biol Pharm Bull. 2011;34(8):1246-51.Furanocoumarin derivatives, known as components of grapefruit juice, showing inhibitory effects against P-glycoprotein (P-gp) in the intestine are also contained in the plants of rutaceae and umbelliferae families, which are used as components of Kampo extract medicines.
In this study, we investigated the inhibitory effects of Byakangelicol and rivulobirin A, known as furanocoumarins showing P-gp inhibitory effect using Caco-2 monolayer, against P-gp at the blood-brain barrier (BBB) under both in vitro and in vivo conditions. |
| Cell Research | Furocoumarins from Angelica dahurica with hepatoprotective activity on tacrine-induced cytotoxicity in Hep G2 cells.[Pubmed: 12058329 ]Planta Med. 2002 May;68(5):463-4.Fractionation of the MeOH extract of Angelica dahurica Benth et Hook resulted in the isolation of six furocoumarins, imperatorin (1), isoimperatorin (2), (+/-)-Byakangelicol (3), (+)-oxypeucedanin (4), (+)-byakangelicin (5), and (+)-aviprin (6).
|

Byakangelicol Dilution Calculator

Byakangelicol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1615 mL | 15.8073 mL | 31.6146 mL | 63.2291 mL | 79.0364 mL |
| 5 mM | 0.6323 mL | 3.1615 mL | 6.3229 mL | 12.6458 mL | 15.8073 mL |
| 10 mM | 0.3161 mL | 1.5807 mL | 3.1615 mL | 6.3229 mL | 7.9036 mL |
| 50 mM | 0.0632 mL | 0.3161 mL | 0.6323 mL | 1.2646 mL | 1.5807 mL |
| 100 mM | 0.0316 mL | 0.1581 mL | 0.3161 mL | 0.6323 mL | 0.7904 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (-)-Oxypeucedanin
Catalog No.:BCC9244
CAS No.:26091-73-6
- Myrciaphenone A
Catalog No.:BCN7003
CAS No.:26089-54-3
- Sotetsuflavone
Catalog No.:BCN3846
CAS No.:2608-21-1
- 3-Isomangostin hydrate
Catalog No.:BCN5134
CAS No.:26063-96-7
- 1-Isomangostin hydrate
Catalog No.:BCN5133
CAS No.:26063-95-6
- Boc-Asp(OBzl)-ONp
Catalog No.:BCC3364
CAS No.:26048-69-1
- Beauvericin
Catalog No.:BCC6546
CAS No.:26048-05-5
- PD173955
Catalog No.:BCC3999
CAS No.:260415-63-2
- Ligustroflavone
Catalog No.:BCN2370
CAS No.:260413-62-5
- Dihydroisotanshinone II
Catalog No.:BCN5132
CAS No.:260397-58-8
- Zamanic acid
Catalog No.:BCN5131
CAS No.:260393-05-3
- GI 254023X
Catalog No.:BCC2374
CAS No.:260264-93-5
- AMC
Catalog No.:BCC2837
CAS No.:26093-31-2
- Otilonium Bromide
Catalog No.:BCC4573
CAS No.:26095-59-0
- Reneilmol
Catalog No.:BCN5135
CAS No.:260968-11-4
- Chicago Sky Blue 6B
Catalog No.:BCC6816
CAS No.:2610-05-1
- Cyanidin-3,5-O-diglucoside chloride
Catalog No.:BCN3116
CAS No.:2611-67-8
- Rhodojaponin II
Catalog No.:BCN2810
CAS No.:26116-89-2
- Picrasin B
Catalog No.:BCN5136
CAS No.:26121-56-2
- Madurensine
Catalog No.:BCN2092
CAS No.:26126-78-3
- 4-O-Feruloylquinic acid
Catalog No.:BCN3352
CAS No.:2613-86-7
- Frentizole
Catalog No.:BCC4035
CAS No.:26130-02-9
- Antiarol rutinoside
Catalog No.:BCN5137
CAS No.:261351-23-9
- MB05032
Catalog No.:BCC1731
CAS No.:261365-11-1
Furocoumarins from Angelica dahurica with hepatoprotective activity on tacrine-induced cytotoxicity in Hep G2 cells.[Pubmed:12058329]
Planta Med. 2002 May;68(5):463-4.
Fractionation of the MeOH extract of Angelica dahurica Benth et Hook resulted in the isolation of six furocoumarins, imperatorin (1), isoimperatorin (2), (+/-)-Byakangelicol (3), (+)-oxypeucedanin (4), (+)-byakangelicin (5), and (+)-aviprin (6). Among these, compounds 1 and 5 exhibited strong hepatoprotective activities, displaying EC(50) values of 36.6 +/- 0.98 and 47.9 +/- 4.6 microM, respectively. Compounds 3 and 4 showed moderate activities with EC(50) values of 112.7 +/- 5.35 and 286.7 +/- 6.36 microM, respectively. Silybin as a positive control showed the EC(50) value with 69.0 +/- 3.4 microM. Comparison of hepatoprotective activities for six furocoumarins 1 - 6 suggested that oxy-substitution at the C-9 position increased the hepatoprotective activity.
Inhibitory effects of furanocoumarin derivatives in Kampo extract medicines on P-glycoprotein at the blood-brain barrier.[Pubmed:21804213]
Biol Pharm Bull. 2011;34(8):1246-51.
Furanocoumarin derivatives, known as components of grapefruit juice, showing inhibitory effects against P-glycoprotein (P-gp) in the intestine are also contained in the plants of rutaceae and umbelliferae families, which are used as components of Kampo extract medicines. In this study, we investigated the inhibitory effects of Byakangelicol and rivulobirin A, known as furanocoumarins showing P-gp inhibitory effect using Caco-2 monolayer, against P-gp at the blood-brain barrier (BBB) under both in vitro and in vivo conditions. First we studied the membrane permeability of furanocoumarins to clarify whether they can be absorbed from the intestine. Both furanocoumarins showed high permeability through the Caco-2 monolayer, suggesting that they can easily reach the systemic circulation after oral administration. Then, we evaluated the effect of these furanocoumarins on the uptake of calcein acetoxymethyl ester (calcein-AM), a P-gp substrate, into bovine brain microvascular endothelial cells (BBMEC). Both furanocoumarins significantly increased the uptake amount of calcein-AM into BBMEC by the inhibition of P-gp at the BBB in vitro. Next we also investigated the P-gp inhibitory effect of these furanocoumarins at the rat BBB in vivo using verapamil as a P-gp substrate. Both furanocoumarins increased the B/P ratio of verapamil compared to the control, even under in vivo conditions; however, the extent of the inhibitory effect was much lower than in vitro condition. In conclusion, Byakangelicol and rivulobirin A may inhibit P-gp expressed at the BBB even under in vivo conditions. Further studies using Kampo extract medicines under in vivo condition are necessary for safe drug therapy.
Byakangelicol, isolated from Angelica dahurica, inhibits both the activity and induction of cyclooxygenase-2 in human pulmonary epithelial cells.[Pubmed:12356282]
J Pharm Pharmacol. 2002 Sep;54(9):1271-8.
We examined the inhibitory mechanism of Byakangelicol, isolated from Angelica dahurica, on interleukin-1beta (IL-1beta)-induced cyclooxygenase-2 (COX-2) expression and prostaglandin E2 (PGE2) release in human pulmonary epithelial cell line (A549). Byakangelicol (10-50 microM) concentration-dependently attenuated IL-1beta-induced COX-2 expression and PGE2 release. The selective COX-2 inhibitor, NS-398 (0.01-1 microM), and Byakangelicol (10-50 microM) both concentration-dependently inhibited the activity of the COX-2 enzyme. Byakangelicol, at a concentration up to 200 microM, did not affect the activity and expression of COX-1 enzyme. IL-1beta-induced p44/42 mitogen-activated protein kinase (MAPK) activation was inhibited by the MAPK/extracellular signal-regulated protein kinase (MEK) inhibitor, PD 98059 (30 microM), while Byakangelicol (50 microM) had no effect. Treatment of cells with Byakangelicol (50 microM) or pyrrolidine dithiocarbamate (PDTC; 50 microM) partially inhibited IL-1beta-induced degradation of IkappaB-alpha in the cytosol, translocation of p65 NF-kappaB from the cytosol to the nucleus and the NF-kappaB-specific DNA-protein complex formation. Taken together, we have demonstrated that Byakangelicol inhibits IL-1beta-induced PGE2 release in A549 cells; this inhibition may be mediated by suppression of COX-2 expression and the activity of COX-2 enzyme. The inhibitory mechanism of Byakangelicol on IL-1beta-induced COX-2 expression may be, at least in part, through suppression of NF-kappaB activity. Therefore, Byakangelicol may have therapeutic potential as an anti-inflammatory drug on airway inflammation.
Antiproliferative effect of furanocoumarins from the root of Angelica dahurica on cultured human tumor cell lines.[Pubmed:17143927]
Phytother Res. 2007 Mar;21(3):288-90.
A bioassay-guided fractionation of the root extract of Angelica dahurica (Umbelliferae) led to the isolation of six furanocoumarins as active ingredients responsible for the antitumoral property. The hexane soluble part of the extract demonstrated a significant inhibition on the proliferation of cultured human tumor cells such as A549 (non small cell lung), SK-OV-3 (ovary), SK-MEL-2 (melanoma), XF498 (central nervous system) and HCT-15 (colon) in vitro, whereas the remaining water soluble part exhibited poor inhibition. Intensive investigation of the hexane soluble part of the extract yielded six furanocoumarins, i.e. isoimperatorin, cnidicin, imperatorin, oxypeucedanin, Byakangelicol, oxypeucedanin hydrate, all of which exhibited a significant inhibition on cell proliferation in a dose-dependent manner.


