MB05032GNG inhibitor,special and efficacious CAS# 261365-11-1 |
- (24R)-MC 976
Catalog No.:BCC1289
CAS No.:112828-09-8
- (24S)-MC 976
Catalog No.:BCC1291
CAS No.:112849-14-6
- 1alpha, 25-Dihydroxy VD2-D6
Catalog No.:BCC1299
CAS No.:216244-04-1
- 1alpha, 24, 25-Trihydroxy VD2
Catalog No.:BCC1298
CAS No.:457048-34-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 261365-11-1 | SDF | Download SDF |
| PubChem ID | 11289630 | Appearance | Powder |
| Formula | C11H15N2O4PS | M.Wt | 302.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (165.40 mM; Need ultrasonic) | ||
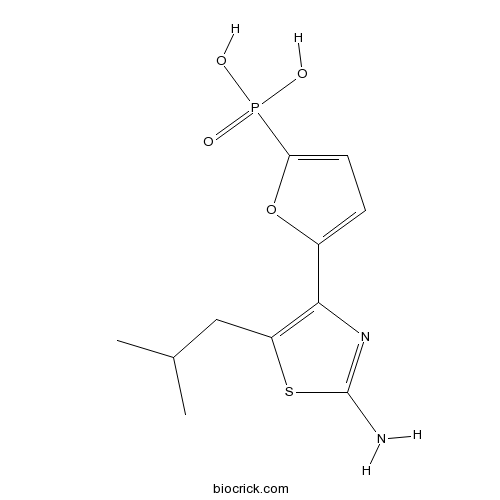
| Chemical Name | [5-[2-amino-5-(2-methylpropyl)-1,3-thiazol-4-yl]furan-2-yl]phosphonic acid | ||
| SMILES | CC(C)CC1=C(N=C(S1)N)C2=CC=C(O2)P(=O)(O)O | ||
| Standard InChIKey | XJMYIJPPDSZOPN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H15N2O4PS/c1-6(2)5-8-10(13-11(12)19-8)7-3-4-9(17-7)18(14,15)16/h3-4,6H,5H2,1-2H3,(H2,12,13)(H2,14,15,16) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | MB05032 is a special and efficacious GNG inhibitor targeted the AMP binding site of fructose 1,6-bisphosphatase (FBPase) with an IC50 value of 16 nM.
IC50 Value: 16 nM (Human Liver FBPase) [1]
Target: Fructose 1, 6-bisphosphatase
Oral delivery of MB05032 was achieved by using the bisamidate prodrug MB06322 (CS-917), which is converted to MB05032 in two steps through the action of an esterase and a phosphoramidase.
in vitro: MB05032 inhibits human liver FBPase with a potency (IC50 = 16 ± 1.5 nM) significantly greater than the natural inhibitor, AMP (IC50 = 1 μM), and the most well characterized AMP mimetic, ZMP (IC50 = 12 ± 1.4 μM). MB05032 inhibits rat FBPase 3-fold weaker (IC50 of 61 ± 4 nM) than human FBPase, whereas AMP is 20-fold weaker as an inhibitor [1]. Inhibition of FBPase activity in islet β-cells by its specific inhibitor MB05032 led to significant increase of their glucose utilization and cellular ATP to ADP ratios and consequently enhanced GSIS in vitro [2].
in vivo: Oral administration of MB06322 to young (8-9 weeks old) ZDF rats with mild diabetes (basal insulin levels of 7.7 ± 0.7 ng/ml) and aged (12-13 weeks) ZDF rats with overt diabetes (basal insulin levels of 0.65 ± 0.16 ng/ml) results in dose-dependent glucose lowering. The dose-response is relatively steep, with 6-10 mg/kg and 30-100 mg/kg being the approximate doses associated with minimal and maximal activity, respectively [1]. Pretreatment of mice with the MB05032 prodrug MB06322 could potentiate GSIS in vivo and improve their glucose tolerance [2].
Toxicity: Neither lactate nor triglycerides increased in 8- to 9-week-old ZDF rats with mild diabetes treated with high doses of MB06322. In ZDF rats with more advanced disease, lactate and triglyceride levels were elevated but only modestly (<2-fold). These results suggest that, unlike inhibitors of other GNG enzymes, FBPase inhibitors may lower glucose with an adequate safety margin [1].
Clinical trial: Evaluation of Glucose Lowering Effect, Safety and Tolerability of CS-917. Phase 2b References: | |||||

MB05032 Dilution Calculator

MB05032 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3081 mL | 16.5404 mL | 33.0808 mL | 66.1616 mL | 82.702 mL |
| 5 mM | 0.6616 mL | 3.3081 mL | 6.6162 mL | 13.2323 mL | 16.5404 mL |
| 10 mM | 0.3308 mL | 1.654 mL | 3.3081 mL | 6.6162 mL | 8.2702 mL |
| 50 mM | 0.0662 mL | 0.3308 mL | 0.6616 mL | 1.3232 mL | 1.654 mL |
| 100 mM | 0.0331 mL | 0.1654 mL | 0.3308 mL | 0.6616 mL | 0.827 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: 16 nM (Human Liver FBPase) [1] MB05032 is a special and efficacious GNG inhibitor targeted the AMP binding site of fructose 1,6-bisphosphatase (FBPase) with an IC50 value of 16 nM. Oral delivery of MB05032 was achieved by using the bisamidate prodrug MB06322 (CS-917), which is converted to MB05032 in two steps through the action of an esterase and a phosphoramidase. in vitro: MB05032 inhibits human liver FBPase with a potency (IC50 = 16 ± 1.5 nM) significantly greater than the natural inhibitor, AMP (IC50 = 1 μM), and the most well characterized AMP mimetic, ZMP (IC50 = 12 ± 1.4 μM). MB05032 inhibits rat FBPase 3-fold weaker (IC50 of 61 ± 4 nM) than human FBPase, whereas AMP is 20-fold weaker as an inhibitor [1]. Inhibition of FBPase activity in islet β-cells by its specific inhibitor MB05032 led to significant increase of their glucose utilization and cellular ATP to ADP ratios and consequently enhanced GSIS in vitro [2]. in vivo: Oral administration of MB06322 to young (8-9 weeks old) ZDF rats with mild diabetes (basal insulin levels of 7.7 ± 0.7 ng/ml) and aged (12-13 weeks) ZDF rats with overt diabetes (basal insulin levels of 0.65 ± 0.16 ng/ml) results in dose-dependent glucose lowering. The dose-response is relatively steep, with 6-10 mg/kg and 30-100 mg/kg being the approximate doses associated with minimal and maximal activity, respectively [1]. Pretreatment of mice with the MB05032 prodrug MB06322 could potentiate GSIS in vivo and improve their glucose tolerance [2]. Toxicity: Neither lactate nor triglycerides increased in 8- to 9-week-old ZDF rats with mild diabetes treated with high doses of MB06322. In ZDF rats with more advanced disease, lactate and triglyceride levels were elevated but only modestly (<2-fold). These results suggest that, unlike inhibitors of other GNG enzymes, FBPase inhibitors may lower glucose with an adequate safety margin [1]. Clinical trial: Evaluation of Glucose Lowering Effect, Safety and Tolerability of CS-917. Phase 2b
- Antiarol rutinoside
Catalog No.:BCN5137
CAS No.:261351-23-9
- Frentizole
Catalog No.:BCC4035
CAS No.:26130-02-9
- 4-O-Feruloylquinic acid
Catalog No.:BCN3352
CAS No.:2613-86-7
- Madurensine
Catalog No.:BCN2092
CAS No.:26126-78-3
- Picrasin B
Catalog No.:BCN5136
CAS No.:26121-56-2
- Rhodojaponin II
Catalog No.:BCN2810
CAS No.:26116-89-2
- Cyanidin-3,5-O-diglucoside chloride
Catalog No.:BCN3116
CAS No.:2611-67-8
- Chicago Sky Blue 6B
Catalog No.:BCC6816
CAS No.:2610-05-1
- Reneilmol
Catalog No.:BCN5135
CAS No.:260968-11-4
- Otilonium Bromide
Catalog No.:BCC4573
CAS No.:26095-59-0
- AMC
Catalog No.:BCC2837
CAS No.:26093-31-2
- Byakangelicol
Catalog No.:BCN5015
CAS No.:26091-79-2
- Linderene
Catalog No.:BCN2779
CAS No.:26146-27-0
- Linderene acetate
Catalog No.:BCN8042
CAS No.:26146-28-1
- 3,5-Dihydroxybenzaldehyde
Catalog No.:BCN2257
CAS No.:26153-38-8
- Naproxen Sodium
Catalog No.:BCC6490
CAS No.:26159-34-2
- CGS 35066
Catalog No.:BCC5916
CAS No.:261619-50-5
- Denudatine
Catalog No.:BCN5406
CAS No.:26166-37-0
- Beesioside Q
Catalog No.:BCC8301
CAS No.:261767-91-3
- 3,19-Dihydroxy-6,23-dioxo-12-ursen-28-oic acid
Catalog No.:BCN1471
CAS No.:261768-88-1
- 6-Methyl-8-prenylnaringenin
Catalog No.:BCN6860
CAS No.:261776-60-7
- 6-Prenylsakuranetin
Catalog No.:BCN7883
CAS No.:261776-61-8
- Foliamenthoic acid
Catalog No.:BCN5138
CAS No.:26187-80-4
- SB269970 HCl
Catalog No.:BCC5056
CAS No.:261901-57-9
Discovery of phosphonic diamide prodrugs and their use for the oral delivery of a series of fructose 1,6-bisphosphatase inhibitors.[Pubmed:18570362]
J Med Chem. 2008 Jul 24;51(14):4331-9.
Like most phosphonic acids, the recently discovered potent and selective thiazole phosphonic acid inhibitors of fructose 1,6-bisphosphatase (FBPase) exhibited low oral bioavailability (OBAV) and therefore required a prodrug to achieve oral efficacy. Syntheses of known phosphonate prodrugs did not afford the desired OBAV; hence, a new class of prodrugs was sought. Phosphonic diamides derived from amino acid esters were discovered as viable prodrugs, which met our preset goals: excellent aqueous stability over a wide pH range, benign byproducts (amino acids and low molecular weight alcohols), and most importantly good OBAV leading to robust oral glucose lowering effects. These desirable properties of phosphonic diamides represent significant improvements over existing prodrug classes. Optimization of the diamide prodrugs of phosphonic acid 2a (MB05032) led to the identification of diamide 8 (MB06322), the first reported orally efficacious FBPase inhibitor.
Fructose-1,6-bisphosphatase regulates glucose-stimulated insulin secretion of mouse pancreatic beta-cells.[Pubmed:20719858]
Endocrinology. 2010 Oct;151(10):4688-95.
Pancreatic beta-cells can precisely sense glucose stimulation and accordingly adjust their insulin secretion. Fructose-1,6-bisphosphatase (FBPase) is a gluconeogenic enzyme, but its physiological significance in beta-cells is not established. Here we determined its physiological role in regulating glucose sensing and insulin secretion of beta-cells. Considerable FBPase mRNA was detected in normal mouse islets and beta-cell lines, although their protein levels appeared to be quite low. Down-regulation of FBP1 in MIN6 cells by small interfering RNA could enhance the glucose-stimulated insulin secretion (GSIS), whereas FBP1-overexpressing MIN6 cells exhibited decreased GSIS. Inhibition of FBPase activity in islet beta-cells by its specific inhibitor MB05032 led to significant increase of their glucose utilization and cellular ATP to ADP ratios and consequently enhanced GSIS in vitro. Pretreatment of mice with the MB05032 prodrug MB06322 could potentiate GSIS in vivo and improve their glucose tolerance. Therefore, FBPase plays an important role in regulating glucose sensing and insulin secretion of beta-cells and serves a promising target for diabetes treatment.
MB06322 (CS-917): A potent and selective inhibitor of fructose 1,6-bisphosphatase for controlling gluconeogenesis in type 2 diabetes.[Pubmed:15911772]
Proc Natl Acad Sci U S A. 2005 May 31;102(22):7970-5.
In type 2 diabetes, the liver produces excessive amounts of glucose through the gluconeogenesis (GNG) pathway and consequently is partly responsible for the elevated glucose levels characteristic of the disease. In an effort to find safe and efficacious GNG inhibitors, we targeted the AMP binding site of fructose 1,6-bisphosphatase (FBPase). The hydrophilic nature of AMP binding sites and their widespread use for allosteric regulation of enzymes in metabolic pathways has historically made discovery of AMP mimetics suitable for drug development difficult. By using a structure-based drug design strategy, we discovered a series of compounds that mimic AMP but bear little structural resemblance. The lead compound, MB05032, exhibited high potency and specificity for human FBPase. Oral delivery of MB05032 was achieved by using the bisamidate prodrug MB06322 (CS-917), which is converted to MB05032 in two steps through the action of an esterase and a phosphoramidase. MB06322 inhibited glucose production from a variety of GNG substrates in rat hepatocytes and from bicarbonate in male Zucker diabetic fatty rats. Analysis of liver GNG pathway intermediates confirmed FBPase as the site of action. Oral administration of MB06322 to Zucker diabetic fatty rats led to a dose-dependent decrease in plasma glucose levels independent of insulin levels and nutritional status. Glucose lowering occurred without signs of hypoglycemia or significant elevations in plasma lactate or triglyceride levels. The findings suggest that potent and specific FBPase inhibitors represent a drug class with potential to treat type 2 diabetes through inhibition of GNG.


