Beauvericinantibacterial and antifungal activities CAS# 26048-05-5 |
- Leupeptin, Microbial
Catalog No.:BCC1217
CAS No.:103476-89-7
- AEBSF.HCl
Catalog No.:BCC1219
CAS No.:30827-99-7
- PMSF
Catalog No.:BCC1229
CAS No.:329-98-6
- Nafamostat Mesylate(FUT-175)
Catalog No.:BCC1228
CAS No.:82956-11-4
- Aprotinin
Catalog No.:BCC1220
CAS No.:9087-70-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 26048-05-5 | SDF | Download SDF |
| PubChem ID | 105014 | Appearance | Powder |
| Formula | C45H57N3O9 | M.Wt | 783.95 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
| Chemical Name | 3,9,15-tribenzyl-4,10,16-trimethyl-6,12,18-tri(propan-2-yl)-1,7,13-trioxa-4,10,16-triazacyclooctadecane-2,5,8,11,14,17-hexone | ||
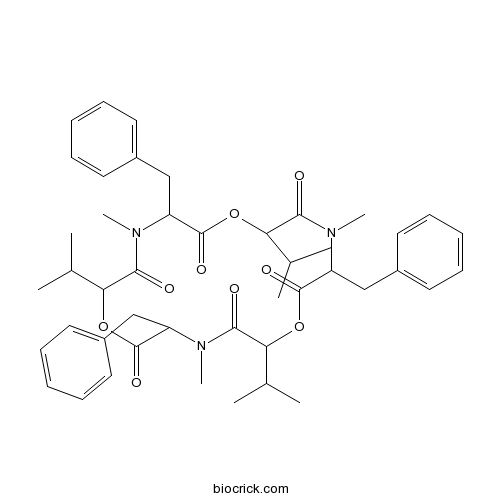
| SMILES | CC(C)C1C(=O)N(C(C(=O)OC(C(=O)N(C(C(=O)OC(C(=O)N(C(C(=O)O1)CC2=CC=CC=C2)C)C(C)C)CC3=CC=CC=C3)C)C(C)C)CC4=CC=CC=C4)C | ||
| Standard InChIKey | GYSCAQFHASJXRS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C45H57N3O9/c1-28(2)37-40(49)46(7)35(26-32-21-15-11-16-22-32)44(53)56-39(30(5)6)42(51)48(9)36(27-33-23-17-12-18-24-33)45(54)57-38(29(3)4)41(50)47(8)34(43(52)55-37)25-31-19-13-10-14-20-31/h10-24,28-30,34-39H,25-27H2,1-9H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Beauvericin Dilution Calculator

Beauvericin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2756 mL | 6.378 mL | 12.7559 mL | 25.5118 mL | 31.8898 mL |
| 5 mM | 0.2551 mL | 1.2756 mL | 2.5512 mL | 5.1024 mL | 6.378 mL |
| 10 mM | 0.1276 mL | 0.6378 mL | 1.2756 mL | 2.5512 mL | 3.189 mL |
| 50 mM | 0.0255 mL | 0.1276 mL | 0.2551 mL | 0.5102 mL | 0.6378 mL |
| 100 mM | 0.0128 mL | 0.0638 mL | 0.1276 mL | 0.2551 mL | 0.3189 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 1.9 μM for antiviral activity; 1.7 μM for antimalarial activity
Beauvericin is a mycotoxin produced by various fungi including Fusarium spp. and Beaveria bassiana. As a cyclic hexadepsipeptide belonging to the enniatin antibiotic family, beauvericin contains three D-hydroxyisovaleryl and three N-methylphenylalanyl residues. Beauvericin was one of the active constituents of B. bassiana and was confirmed to have antibacterial and antifungal activities.
In vitro: Beauvericin has a strong antibacterial activity against human, animal and plant pathogenic bacteria, without selectivity between Gram-positive and Gram-negative bacteria. Unlike other antibiotics blocking the peptidoglycan biosynthesis, beauvericin does not target on the bacterial cell wall. Although it has broad-spectrum antibacterial activities, the antifungal activity of beauvericin is rarely reported as a single agent. Thus, the target of beauvericin is regarded to be different between bacteria and fungi and the activities of beauvericin need to be investigated against drug resistant bacteria [1].
In vivo: Previous study reported the the antifungal activity of beauvericin combined with miconazole or ketoconazole. Beauvericin at 0.5 mg/kg combined with ketoconazole at 0.5 mg/kg showed significant antifungal activity against Candida parapsilosis, which could cause high mortality rates quickly, particularly in neonates. In contrast, both beauvericin and ketoconazole alone have little to no inhibitory effect on C. parapsilosis [1].
Clinical trial: N/A
Reference:
[1] Wang Q, Xu L. Beauvericin, a bioactive compound produced by fungi: a short review. Molecules. 2012 Feb 24;17(3):2367-77.
- PD173955
Catalog No.:BCC3999
CAS No.:260415-63-2
- Ligustroflavone
Catalog No.:BCN2370
CAS No.:260413-62-5
- Dihydroisotanshinone II
Catalog No.:BCN5132
CAS No.:260397-58-8
- Zamanic acid
Catalog No.:BCN5131
CAS No.:260393-05-3
- GI 254023X
Catalog No.:BCC2374
CAS No.:260264-93-5
- Xylose
Catalog No.:BCC4880
CAS No.:25990-60-7
- Nimbolide
Catalog No.:BCN8053
CAS No.:25990-37-8
- 4-Hydroxythiobenzamide
Catalog No.:BCC8709
CAS No.:25984-63-8
- Hyponine D
Catalog No.:BCC8998
CAS No.:259823-31-9
- T 705
Catalog No.:BCC4130
CAS No.:259793-96-9
- 2-Deacetyltaxuspine X
Catalog No.:BCN7375
CAS No.:259678-73-4
- 5-Hydroxy-7-methoxy-3-(4-hydroxybenzylidene)chroman-4-one
Catalog No.:BCN1472
CAS No.:259653-54-8
- Boc-Asp(OBzl)-ONp
Catalog No.:BCC3364
CAS No.:26048-69-1
- 1-Isomangostin hydrate
Catalog No.:BCN5133
CAS No.:26063-95-6
- 3-Isomangostin hydrate
Catalog No.:BCN5134
CAS No.:26063-96-7
- Sotetsuflavone
Catalog No.:BCN3846
CAS No.:2608-21-1
- Myrciaphenone A
Catalog No.:BCN7003
CAS No.:26089-54-3
- (-)-Oxypeucedanin
Catalog No.:BCC9244
CAS No.:26091-73-6
- Byakangelicol
Catalog No.:BCN5015
CAS No.:26091-79-2
- AMC
Catalog No.:BCC2837
CAS No.:26093-31-2
- Otilonium Bromide
Catalog No.:BCC4573
CAS No.:26095-59-0
- Reneilmol
Catalog No.:BCN5135
CAS No.:260968-11-4
- Chicago Sky Blue 6B
Catalog No.:BCC6816
CAS No.:2610-05-1
- Cyanidin-3,5-O-diglucoside chloride
Catalog No.:BCN3116
CAS No.:2611-67-8
In vitro effects of the Fusarium mycotoxins fumonisin B1 and beauvericin on bovine granulosa cell proliferation and steroid production.[Pubmed:28132864]
Toxicon. 2017 Mar 15;128:38-45.
Fusarium mycotoxins are natural contaminants of various commodities representing significant problem worldwide. Since the co-occurrence of Beauvericin (BEA) and fumonisin B1 (FB1) in grains is frequent, the present study was carried out to evaluate the individual and combined effects of FB1 and BEA on cell proliferation, steroid production and gene expression using bovine granulosa cells (GC). When tested alone FB1 did not show (P >/= 0.05) effects on cell proliferation at any dose. Whereas BEA at 3, 6, and 10 muM significantly decreased (P < 0.05) cell numbers. FB1 alone had no significant effect (P >/= 0.05) on progesterone production at any tested doses, whereas FB1 at 1, 1.5 and 3 muM slightly inhibited (P < 0.05) estradiol production. At concentrations >/=3 muM, BEA was found to strongly decrease (P < 0.05) steroid production, and FB1 did not influence these effects of BEA. At 10 muM, both mycotoxins decreased (P < 0.001) serum-induced GC proliferation. At 30 muM, BEA showed inhibitory effects on FSH plus IGF1-induced CYP11A1 and CYP19A1 mRNA abundance (P < 0.05), whereas FB1 at 30 muM had no effect on CYP11A1 and CYP19A1 gene expression. Taken together these results demonstrate that the Fusarium mycotoxins BEA and FB1 may impair reproductive function in cattle.
Enniatin and Beauvericin Biosynthesis in Fusarium Species: Production Profiles and Structural Determinant Prediction.[Pubmed:28125067]
Toxins (Basel). 2017 Jan 25;9(2). pii: toxins9020045.
Members of the fungal genus Fusarium can produce numerous secondary metabolites, including the nonribosomal mycotoxins Beauvericin (BEA) and enniatins (ENNs). Both mycotoxins are synthesized by the multifunctional enzyme enniatin synthetase (ESYN1) that contains both peptide synthetase and S-adenosyl-l-methionine-dependent N-methyltransferase activities. Several Fusarium species can produce ENNs, BEA or both, but the mechanism(s) enabling these differential metabolic profiles is unknown. In this study, we analyzed the primary structure of ESYN1 by sequencing esyn1 transcripts from different Fusarium species. We measured ENNs and BEA production by ultra-performance liquid chromatography coupled with photodiode array and Acquity QDa mass detector (UPLC-PDA-QDa) analyses. We predicted protein structures, compared the predictions by multivariate analysis methods and found a striking correlation between BEA/ENN-producing profiles and ESYN1 three-dimensional structures. Structural differences in the beta strand's Asn789-Ala793 and His797-Asp802 portions of the amino acid adenylation domain can be used to distinguish BEA/ENN-producing Fusarium isolates from those that produce only ENN.
Development and validation of ultra-high-performance liquid chromatography-tandem mass spectrometry methods for the simultaneous determination of beauvericin, enniatins (A, A1, B, B1) and cereulide in maize, wheat, pasta and rice.[Pubmed:27776774]
J Chromatogr A. 2016 Nov 11;1472:35-43.
Rapid and accurate UPLC-MS/MS methods for the simultaneous determination of Beauvericin and the related enniatins (A, A1, B, B1), together with cereulide were successfully developed and validated in cereal and cereal-based food matrices such as wheat, maize, rice and pasta. Although these emerging foodborne toxins are of different microbial origin, the similar structural, toxicological and food safety features provided rationale for their concurrent detection in relevant food matrices. A Waters Acquity UPLC system coupled to a Waters Quattro Premier XE Mass Spectrometer operating in ESI+ mode was employed. Sample pretreatment involved a fast and simple liquid extraction of the target toxins without any further clean-up step. For all toxins the sample preparation resulted in acceptable extraction recoveries with values of 85-105% for wheat, 87-106% for maize, 84-106% for rice and 85-105% for pasta. The efficient extraction protocol, together with a fast chromatographic separation of 7min allowed substantial saving costs and time showing its robustness and performance. The validation of the developed method was performed based on Commission Decision 2002/657/EC. The obtained limits of detection ranged from 0.1 to 1.0mugkg(-1) and the limits of quantification from 0.3 to 2.9mugkg(-1) for the targeted toxins in the selected matrices. The obtained sensitivities allow detection of relevant toxicological concentrations. All relative standard deviations for repeatability (intra-day) and intermediate precision (inter-day) were lower than 20%. Trueness, expressed as the apparent recovery varied from 80 to 107%. The highly sensitive and repeatable validated method was applied to 57 naturally contaminated samples allowing detection of sub-clinical doses of the toxins.
Beauvericin Potentiates Azole Activity via Inhibition of Multidrug Efflux, Blocks Candida albicans Morphogenesis, and Is Effluxed via Yor1 and Circuitry Controlled by Zcf29.[Pubmed:27736764]
Antimicrob Agents Chemother. 2016 Nov 21;60(12):7468-7480.
Invasive fungal infections are a leading cause of human mortality. Effective treatment is hindered by the rapid emergence of resistance to the limited number of antifungal drugs, demanding new strategies to treat life-threatening fungal infections. Here, we explore a powerful strategy to enhance antifungal efficacy against leading human fungal pathogens by using the natural product Beauvericin. We found that Beauvericin potentiates the activity of azole antifungals against azole-resistant Candida isolates via inhibition of multidrug efflux and that Beauvericin itself is effluxed via Yor1. As observed in Saccharomyces cerevisiae, we determined that Beauvericin inhibits TOR signaling in Candida albicans To further characterize Beauvericin activity in C. albicans, we leveraged genome sequencing of Beauvericin-resistant mutants. Resistance was conferred by mutations in transcription factor genes TAC1, a key regulator of multidrug efflux, and ZCF29, which was uncharacterized. Transcriptional profiling and chromatin immunoprecipitation coupled to microarray analyses revealed that Zcf29 binds to and regulates the expression of multidrug transporter genes. Beyond drug resistance, we also discovered that Beauvericin blocks the C. albicans morphogenetic transition from yeast to filamentous growth in response to diverse cues. We found that Beauvericin represses the expression of many filament-specific genes, including the transcription factor BRG1 Thus, we illuminate novel circuitry regulating multidrug efflux and establish that simultaneously targeting drug resistance and morphogenesis provides a promising strategy to combat life-threatening fungal infections.


